Mastering the SLU-pp-332 Stack: Best Peptides for Enhanced Research in 2025
The pursuit of optimizing biological systems for enhanced wellness and performance is a perpetual journey for researchers. In this quest, certain compounds emerge as significant subjects of study, drawing considerable attention for their potential. Among these, SLU-pp-332 has garnered substantial interest, particularly when considering its role in a well-constructed peptide stack. As we delve into 2025, understanding how to effectively incorporate SLU-pp-332 and identify the best peptides to use alongside it is crucial for maximizing research outcomes. This comprehensive guide aims to illuminate the intricate world of SLU-pp-332 and its synergistic partners, providing researchers with the insights needed to design potent and effective experimental protocols.
Key Takeaways
- SLU-pp-332 is a novel peptide with significant research potential, primarily focused on metabolic and muscle-related pathways.
- Stacking SLU-pp-332 with other peptides can create synergistic effects, amplifying individual benefits and addressing multiple biological targets simultaneously.
- Key peptides often considered for a SLU-pp-332 stack include MOTS-c, BPC-157, Epitalon, and various growth hormone-releasing peptides.
- Careful consideration of research objectives, potential interactions, and responsible sourcing (e.g., from reputable vendors like Pure Tested Peptides) is paramount for successful peptide stacking.
- Emerging research in 2025 continues to unveil new insights into optimal SLU-pp-332 peptide combinations and their applications.
Unpacking SLU-pp-332: Mechanisms and Research Potential
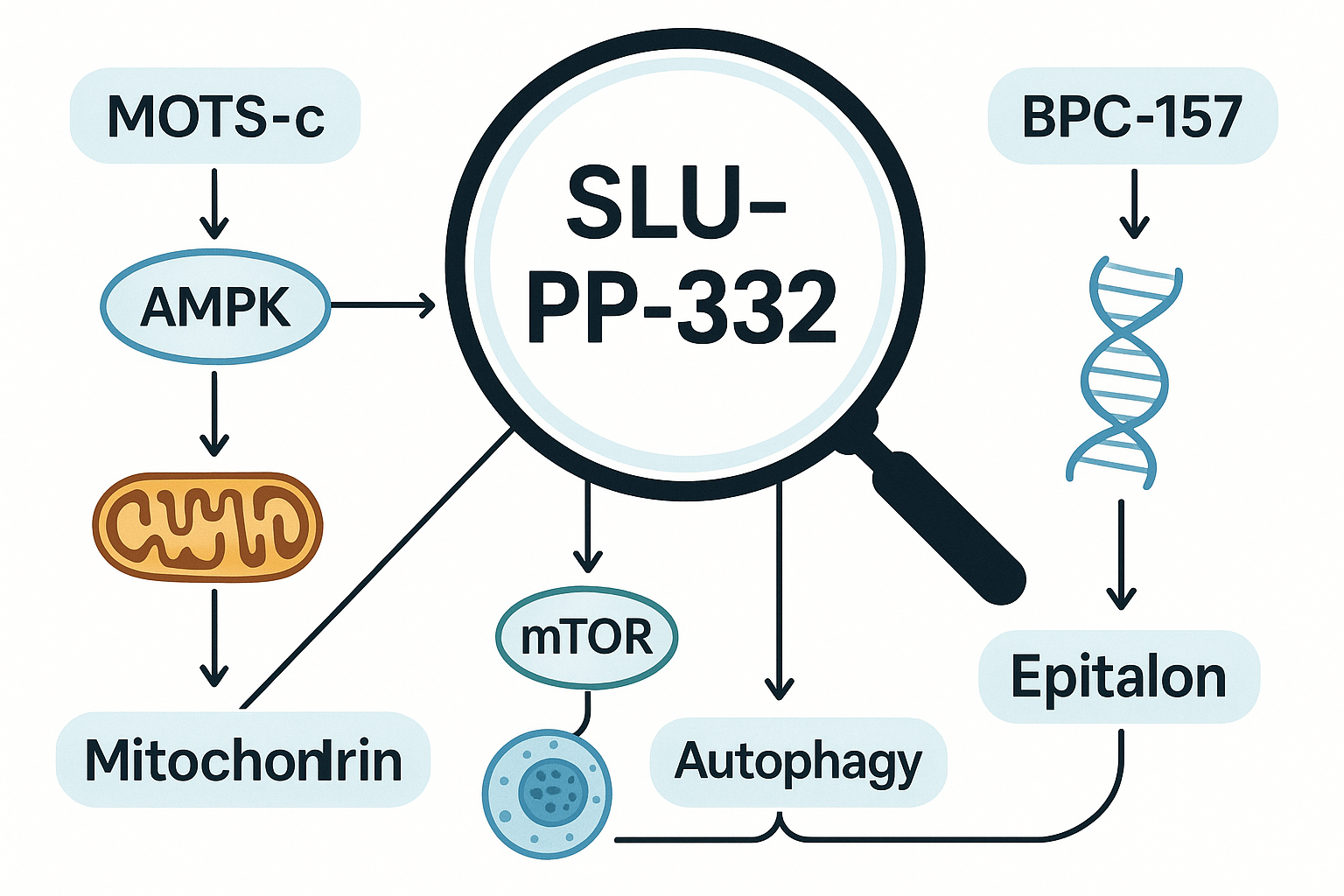
The journey into the world of peptides often begins with understanding the individual components. SLU-pp-332, a relatively new contender in the research peptide landscape, has quickly captured attention due to its intriguing biological profile. At its core, SLU-pp-332 is a synthetic peptide derived from the sarcolipin protein, a small regulatory peptide found in muscle tissue. Sarcolipin plays a crucial role in regulating calcium handling within muscle cells, particularly impacting the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) pump. This pump is vital for muscle contraction and relaxation, as well as thermogenesis.
Researchers are particularly interested in SLU-pp-332 because it appears to modulate SERCA activity, specifically uncoupling it from ATP hydrolysis. This uncoupling mechanism is theorized to increase heat production and energy expenditure within muscle cells, without necessarily leading to mechanical work. Imagine a furnace that burns fuel more efficiently, but instead of producing more power, it primarily produces more heat. This has profound implications for metabolic research, especially in areas related to energy balance, fat oxidation, and thermogenesis.
One of the primary areas of research for SLU-pp-332 involves its potential impact on:
- Metabolic Rate: By increasing energy expenditure at a cellular level, SLU-pp-332 could be a subject of study for understanding metabolic disorders.
- Fat Oxidation: Enhanced thermogenesis often correlates with increased fat burning, making it a target for research into body composition management.
- Muscle Performance and Adaptation: While not directly anabolic in the traditional sense, its influence on muscle energetics could play a role in how muscles adapt to different physiological stressors.
Anecdotally, one researcher shared their initial skepticism about a peptide focused solely on thermogenesis. “We were so focused on direct muscle growth,” they recounted, “that the idea of a compound improving efficiency in a less direct way seemed almost too subtle. But once we started observing the cellular energy shifts, the picture became much clearer. It’s like optimizing the engine’s fuel efficiency rather than just adding more horsepower.” This perspective highlights the nuanced approach required when studying SLU-pp-332.
The mechanism of action for SLU-pp-332 is distinct from many other well-known peptides. It doesn’t primarily act as a growth factor, a hormone secretagogue, or a direct tissue repair agent. Instead, it seems to fine-tune an internal metabolic process, making it a unique addition to the research peptide toolkit. This distinct mechanism is precisely what makes the SLU-pp-332 stack so compelling for researchers seeking multi-faceted approaches to their studies. When sourcing such peptides, always prioritize quality and transparency. Reputable suppliers like Pure Tested Peptides ensure product integrity, which is paramount for reliable research.
Why Consider a SLU-pp-332 Stack?
The concept of “stacking” peptides isn’t about simply combining multiple compounds; it’s about leveraging synergy. Synergy occurs when the combined effect of two or more agents is greater than the sum of their individual effects. In the context of a SLU-pp-332 stack, researchers aim to:
- Amplify Benefits: If SLU-pp-332 enhances metabolic efficiency, combining it with a peptide that supports muscle anabolism could lead to a more profound impact on body composition.
- Target Multiple Pathways: A single peptide typically targets a specific pathway. A stack allows researchers to address several biological processes simultaneously, offering a more holistic approach to complex research questions. For instance, addressing both metabolic health and recovery.
- Mitigate Limitations: Some peptides might have specific limitations or side effects that can be counteracted or balanced by another peptide in the stack.
- Explore Novel Interactions: Sometimes, unexpected positive interactions can emerge from specific combinations, opening new avenues for discovery.
Considering these points, designing a SLU-pp-332 peptide stack requires a deep understanding of each component’s mechanism of action and how they might interact. It’s not unlike a culinary chef carefully selecting ingredients to create a harmonious and impactful dish. Each ingredient, or peptide, brings its own unique flavor profile and properties, and the right combination can elevate the entire experience.
Best Peptides to Stack with SLU-pp-332 for Optimal Research Outcomes
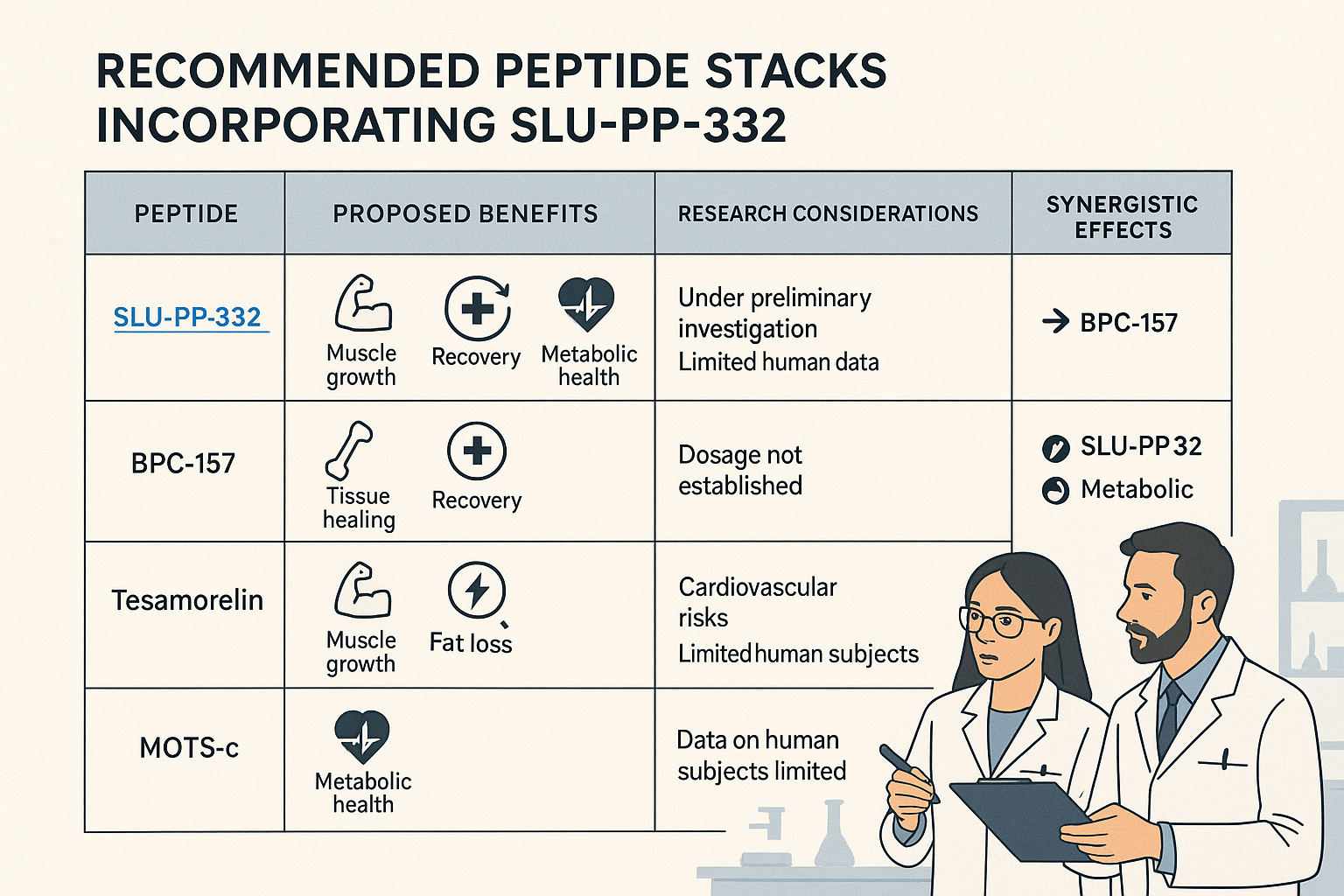
When building a SLU-pp-332 stack, the goal is often to create a comprehensive research model that addresses various aspects of physiological function. The choice of companion peptides will depend heavily on the specific research objective. Here are some of the most compelling peptides to consider stacking with SLU-pp-332, along with their rationales and potential synergies.
1. MOTS-c: The Metabolic Powerhouse
MOTS-c (Mitochondrial-derived peptide of 16 amino acids) is perhaps one of the most obvious and powerful choices for a SLU-pp-332 stack. Why? Because both peptides are deeply involved in metabolic regulation, albeit through different mechanisms.
- MOTS-c’s Role: MOTS-c primarily influences mitochondrial function, promoting insulin sensitivity, glucose metabolism in skeletal muscle, and overall energy homeostasis. It’s often referred to as an “exercise-mimetic” due to its ability to induce some of the metabolic benefits typically associated with physical activity. It helps cells use glucose more efficiently and can protect against diet-induced insulin resistance.
- Synergy with SLU-pp-332: While SLU-pp-332 focuses on thermogenesis and energy expenditure via SERCA uncoupling, MOTS-c optimizes glucose utilization and mitochondrial efficiency. This combination could offer a two-pronged attack on metabolic dysregulation:
- Enhanced Energy Utilization: SLU-pp-332 increases overall caloric burn, while MOTS-c ensures that the available energy (especially glucose) is processed and utilized effectively by the mitochondria.
- Improved Insulin Sensitivity: MOTS-c directly improves insulin sensitivity, which, when combined with SLU-pp-332‘s potential to reduce fat stores through thermogenesis, could lead to a more favorable metabolic profile.
- Anti-diabetic Research: This stack holds significant promise for research into metabolic disorders like type 2 diabetes, where both insulin resistance and impaired energy metabolism are central issues.
Consider a research scenario where understanding the full spectrum of metabolic improvement is key. A study combining these two peptides could observe a more pronounced effect on glucose disposal and fat loss than either peptide alone.
2. BPC-157: The Healing and Recovery Agent
BPC-157 (Body Protection Compound-157) is a fascinating gastric pentadecapeptide known for its remarkable regenerative and protective properties. While its primary mechanisms are distinct from SLU-pp-332, the synergy lies in supporting overall physiological resilience during metabolic stress.
- BPC-157’s Role: BPC-157 is renowned for its ability to accelerate wound healing (e.g., tendon, ligament, muscle), reduce inflammation, protect organs (like the stomach and liver), and promote angiogenesis (formation of new blood vessels). It also appears to influence neurotransmitter systems, offering neuroprotective effects. You can find high-quality BPC-157 for research purposes at Pure Tested Peptides.
- Synergy with SLU-pp-332: If SLU-pp-332 is increasing metabolic demand and potentially inducing thermogenic stress, BPC-157 could serve as a vital protective and recovery element within the SLU-pp-332 stack.
- Counteracting Stress: Intense metabolic shifts can sometimes induce physiological stress. BPC-157’s anti-inflammatory and cytoprotective effects could help mitigate any adverse reactions or promote faster adaptation.
- Tissue Repair and Adaptation: For studies involving muscle adaptation to increased energy expenditure, BPC-157 could facilitate faster repair of micro-traumas and improved tissue integrity, complementing SLU-pp-332‘s metabolic influence.
- Gut Health: BPC-157’s benefits to gut integrity are well-documented. A healthy gut is crucial for nutrient absorption and overall metabolic health, indirectly supporting the goals of a SLU-pp-332 peptide study.
Imagine a research project examining exercise performance and metabolic efficiency. While SLU-pp-332 could optimize energy burn, BPC-157 could ensure that the recovery and adaptive processes of the muscles and connective tissues are maximized, leading to more robust and sustainable gains in performance indicators. More information on BPC-157 research themes is available.
3. Epitalon: The Longevity and Regenerative Cofactor
Epitalon (Epithalamin) is a synthetic tetrapeptide derived from the pineal gland, widely studied for its potential anti-aging and regenerative properties. Its inclusion in a SLU-pp-332 stack offers a long-term, systemic benefit that complements the acute metabolic effects of SLU-pp-332.
- Epitalon’s Role: Epitalon is primarily known for influencing the pineal gland, leading to increased melatonin production, regulation of circadian rhythms, and modulation of various endocrine functions. It has been shown to lengthen telomeres, which are protective caps on chromosomes, thereby potentially slowing cellular aging. Its impact on antioxidant enzymes and immune function is also under investigation. You can explore Epitalon peptides for sale for your research needs.
- Synergy with SLU-pp-332: While SLU-pp-332 optimizes metabolic processes, Epitalon provides a foundational layer of cellular health and longevity, enhancing the overall physiological environment.
- Cellular Resilience: If SLU-pp-332 is pushing metabolic boundaries, Epitalon’s telomere-lengthening and antioxidant properties could help maintain cellular health and function, preventing oxidative stress or cellular senescence.
- Improved Sleep and Recovery: Epitalon’s influence on melatonin production can significantly improve sleep quality. Adequate sleep is critical for metabolic recovery, hormone regulation, and overall well-being, directly supporting the metabolic goals of a SLU-pp-332 peptide study.
- Systemic Optimization: This stack moves beyond localized effects to a more systemic approach. SLU-pp-332 handles the metabolic “engine,” while Epitalon ensures the entire “vehicle” (the organism) is in optimal condition for the long haul. More on Epitalon longevity signals can be found here.
A long-term study looking at metabolic health and markers of aging would greatly benefit from this combination. The acute metabolic benefits of SLU-pp-332 combined with Epitalon’s cellular maintenance could provide a robust model for understanding healthy aging processes.
4. Growth Hormone-Releasing Peptides (GHRPs) and GHRH Analogs
Peptides like CJC-1295 (with or without DAC) and Ipamorelin are classified as Growth Hormone-Releasing Hormones (GHRHs) and Growth Hormone-Releasing Peptides (GHRPs), respectively. They stimulate the body’s natural production and pulsatile release of growth hormone (GH).
- GHRPs/GHRH Analogs’ Role: These peptides work by binding to specific receptors in the pituitary gland, leading to an increased secretion of endogenous growth hormone. GH is crucial for muscle growth, fat metabolism, tissue repair, and overall body composition. CJC-1295 and Ipamorelin are often stacked together for synergistic GH release.
- Synergy with SLU-pp-332: This combination targets both metabolic efficiency and anabolic drive, creating a powerful research model for body composition and recovery.
- Enhanced FL: SLU-pp-332 can boost energy expenditure and fat oxidation, while GH directly mobilizes fat stores for energy. This dual action could lead to more profound reductions in adipose tissue.
- Muscle Preservation/Growth: While SLU-pp-332 is not directly anabolic, increased GH levels contribute to muscle protein synthesis and repair, helping to preserve or even build muscle mass, especially in a caloric deficit where SLU-pp-332 might be employed for fat loss research.
- Improved Recovery: GH also plays a critical role in tissue repair and recovery from physiological stress, which would complement any intense metabolic shifts induced by SLU-pp-332. Research on CJC-1295 and Ipamorelin synergy further elaborates on this.
A research protocol focusing on optimizing body composition for athletes or individuals with metabolic challenges would find this SLU-pp-332 stack particularly relevant. The combination of increased energy expenditure and enhanced anabolic/recovery signals could lead to significant and measurable changes in lean mass to fat mass ratios. For those delving into detailed studies, exploring the differences between CJC-1295 with and without DAC is also recommended.
5. 5-Amino-1MQ: NAD+ Optimization for Cellular Energy
5-Amino-1MQ is a small molecule that inhibits nicotinamide N-methyltransferase (NNMT), an enzyme that plays a role in NAD+ metabolism. By inhibiting NNMT, 5-Amino-1MQ is thought to increase NAD+ levels within cells.
- 5-Amino-1MQ’s Role: NAD+ is a crucial coenzyme involved in countless metabolic processes, including energy production (ATP), DNA repair, and sirtuin activation (which are linked to longevity). By potentially boosting NAD+ levels, 5-Amino-1MQ can enhance cellular energy and metabolic function. More information on 5-Amino-1MQ is available for researchers.
- Synergy with SLU-pp-332: This stack focuses on two distinct but complementary aspects of energy metabolism.
- Fueling the Thermogenic Process: If SLU-pp-332 is pushing the metabolic furnace to burn more efficiently, 5-Amino-1MQ could ensure there’s ample NAD+ “fuel” for all the associated enzymatic reactions and energy production.
- Overall Cellular Energetics: Combining a peptide that influences specific energy expenditure pathways (SLU-pp-332) with a compound that boosts the fundamental energy currency of the cell (NAD+ via 5-Amino-1MQ) could lead to a highly optimized metabolic state.
- Mitochondrial Support: Both compounds indirectly support mitochondrial function – SLU-pp-332 by influencing energy dissipation, and 5-Amino-1MQ by providing essential cofactors for oxidative phosphorylation. Researchers can explore 5-Amino-1MQ peptides for sale to incorporate this into their studies.
This stack would be highly relevant for research into chronic fatigue, metabolic syndrome, and any condition where cellular energy deficits are a contributing factor. The combined action could lead to measurable improvements in cellular vitality and metabolic efficiency.
Considerations for Designing Your SLU-pp-332 Peptide Stack
When designing any peptide stack, especially one involving a potent compound like SLU-pp-332, several critical factors must be taken into account:
- Research Objectives: Clearly define what you aim to achieve with the stack. Are you targeting fat loss, muscle growth, metabolic health, recovery, or a combination? Your objectives will dictate the choice of peptides.
- Dosage and Administration: Each peptide has its own optimal research dosage and administration route. These need to be carefully considered when combining peptides to ensure efficacy and minimize any potential interactions. Refer to established research protocols and supplier guidelines.
- Timing: The timing of peptide administration can be crucial. Some peptides are best administered in the morning, others before bed, and some around exercise. Harmonizing the timing within a stack is important. For example, daily routines and peptide timing are vital for effective research.
- Sourcing and Quality: Always purchase peptides from reputable suppliers who provide third-party testing and Certificates of Analysis (CoAs). The purity and authenticity of your research materials are paramount for valid and reproducible results. Pure Tested Peptides is committed to providing high-quality, verified research peptides. You can even check their CoA for product verification.
- Monitoring and Data Collection: When using a stack, comprehensive monitoring of relevant biomarkers, physiological parameters, and observable effects is essential. This allows researchers to gauge the efficacy of the stack and make informed adjustments. Building reproducible wellness studies depends on rigorous data collection.
- Ethical Considerations: Ensure all research involving peptides adheres to ethical guidelines and regulations. These compounds are strictly for research purposes and not for human consumption.
A researcher once shared a cautionary tale about an ill-conceived stack. “We got excited about combining five different peptides, thinking ‘more is better,'” they explained. “But we ended up with conflicting signals and couldn’t isolate the effects. It taught us a valuable lesson: start small, understand the synergy, and build methodically. It’s like trying to listen to five different conversations at once – you hear noise, not insight.” This anecdote underscores the importance of a thoughtful, scientific approach to stacking.
Emerging Trends in 2025 for SLU-pp-332 Stacks
As we progress through 2025, the research landscape for peptides, and specifically for SLU-pp-332, continues to evolve. New discoveries are constantly being made regarding peptide interactions and optimal research applications.
One notable area of growing interest is the combination of SLU-pp-332 with compounds aimed at improving adaptive capacity. The notion of the body’s ability to respond and adapt to various stressors is gaining traction, and peptides that enhance this capacity are becoming more relevant. For instance, researchers are exploring how SLU-pp-332‘s metabolic efficiency could synergize with peptides that support stress resilience or cognitive function, providing a more robust model for overall wellness research. Adaptive capacity and peptide mapping are increasingly important areas of study.
Another trend is the focus on personalized research models. Instead of one-size-fits-all stacks, the emphasis is shifting towards tailoring peptide combinations based on specific experimental goals and observed baseline trends. This requires a deeper understanding of individual peptide pharmacology and how they interact within complex biological systems. Leveraging tools for baseline trends and data quality is crucial here.
Finally, the exploration of novel delivery methods for peptides is always ongoing. While injectable forms remain standard for many research applications, advancements in oral, nasal, and transdermal peptide research could significantly broaden the scope and ease of conducting studies with peptides like SLU-pp-332.
The dynamic nature of peptide research means that staying informed about the latest findings and continually evaluating new combinations is key. The scientific community is a vibrant place, and the conversations surrounding a powerful SLU-pp-332 peptide and its potential synergies are only just beginning.
Conclusion
The landscape of peptide research in 2025 is rich with potential, and SLU-pp-332 stands out as a particularly exciting compound for metabolic investigation. Its unique mechanism of enhancing thermogenesis and energy expenditure through SERCA uncoupling offers a novel pathway for impacting metabolic health and body composition. However, the true power of SLU-pp-332 is often unlocked when it is intelligently integrated into a synergistic peptide stack.
By combining SLU-pp-332 with peptides like MOTS-c for enhanced metabolic efficiency, BPC-157 for robust recovery and tissue repair, Epitalon for cellular longevity and systemic health, GHRPs/GHRH analogs for anabolic drive and fat mobilization, or 5-Amino-1MQ for foundational cellular energy, researchers can create comprehensive and targeted experimental models. These stacks allow for a multi-faceted approach, addressing various biological pathways simultaneously and potentially yielding more profound and holistic insights.
For any researcher embarking on studies involving SLU-pp-332 or other research peptides, the foundational principles remain paramount: thorough understanding of each peptide’s mechanism, meticulous experimental design, careful consideration of dosages and timing, and an unwavering commitment to sourcing high-quality, verified compounds from reputable suppliers like Pure Tested Peptides.
As the scientific community continues to uncover the intricate dance of peptides within biological systems, the judicious use of a SLU-pp-332 stack promises to be a powerful tool for advancing our understanding of metabolic health, performance, and longevity in 2025 and beyond.
Actionable Next Steps for Researchers:
- Define Your Hypothesis: Clearly articulate the specific research question your SLU-pp-332 stack aims to answer.
- Select Your Stack Components: Based on your hypothesis, choose peptides from the discussed options (MOTS-c, BPC-157, Epitalon, GHRPs/GHRH analogs, 5-Amino-1MQ) that offer complementary mechanisms to SLU-pp-332.
- Consult Existing Literature: Dive deep into current research on each selected peptide and their known interactions. Look for studies on comparing single peptides and multi-peptide blends.
- Source Wisely: Obtain all peptides from a trusted vendor like Pure Tested Peptides to ensure purity and authenticity.
- Design Your Protocol: Develop a detailed experimental protocol, including dosages, administration routes, timing, and monitoring parameters. Remember the best practices for storing research peptides.
- Start with Caution: Begin with conservative doses and observe effects meticulously. Incremental adjustments are key to successful peptide research.
Meta Title: SLU-pp-332 Stack: Best Peptides for Research in 2025
Meta Description: Explore optimal SLU-pp-332 peptide stacks for advanced research in 2025. Discover synergistic peptides like MOTS-c, BPC-157, and Epitalon for enhanced metabolic and wellness studies.
