Sending Peptides for Purity Testing: Ensuring Quality and Achieving 99% Purity Peptides in 2025
In the dynamic world of biochemical research, the integrity of your experimental results hinges on one critical factor: the purity of your reagents. For researchers working with peptides, the importance of robust peptide purity testing cannot be overstated. Imagine spending weeks or months on a groundbreaking study, only to discover that your peptide samples contained impurities that skewed your findings. This scenario, unfortunately, is a real risk if you don’t prioritize testing peptide purity with certified methods. As we navigate 2025, the demand for reliable, high-quality research materials, particularly those guaranteeing 99% purity peptides, continues to escalate. This comprehensive guide will walk you through everything you need to know about sending peptides for purity testing, ensuring your research stands on a foundation of uncompromised quality.
Key Takeaways
- Purity is Paramount: Impurities in peptides can severely compromise research integrity, leading to inaccurate results and wasted resources.
- Gold Standard Methods: High-Performance Liquid Chromatography (HPLC) and Mass Spectrometry (MS) are the primary techniques for accurate peptide purity assessment.
- Understanding the CoA: A Certificate of Analysis (CoA) is your assurance of quality, detailing purity levels, molecular weight, and any detected contaminants.
- Choosing a Reputable Lab: Selecting a certified, experienced laboratory for peptide purity testing is crucial for reliable and reproducible results.
- Proper Sample Preparation: Correct handling, packaging, and shipping are vital to maintain peptide stability and prevent degradation during transit.
The Critical Importance of Peptide Purity Testing for Research Integrity
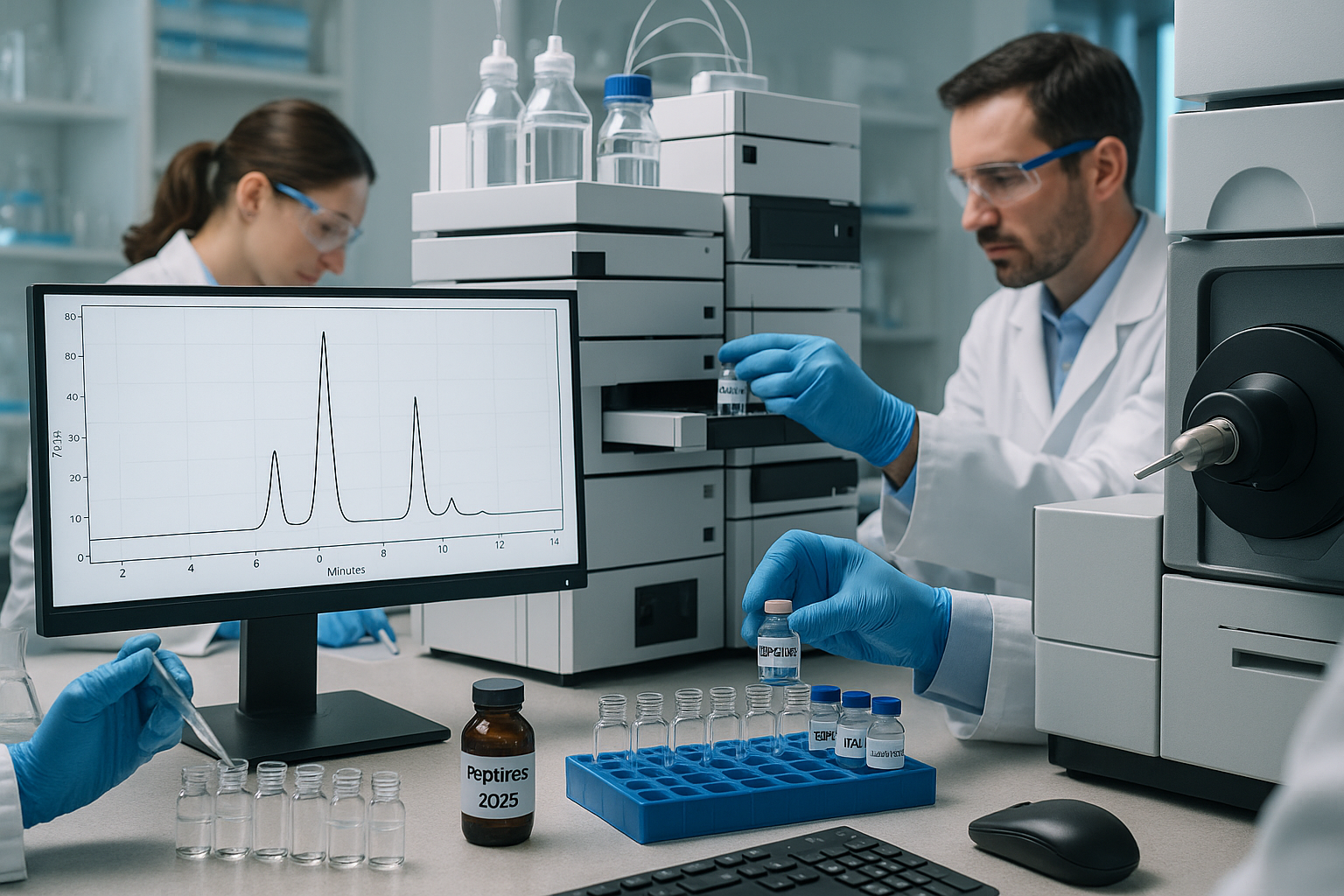
The scientific landscape of 2025 is characterized by an increasing drive for precision and reproducibility. In this environment, the quality of your peptide samples is not just a preference; it’s a fundamental requirement. Peptides, as short chains of amino acids, are complex molecules used in a vast array of research, from drug discovery and development to understanding cellular processes. Their specific biological activity is often highly dependent on their exact amino acid sequence and structural integrity. Even minor impurities can drastically alter how a peptide behaves in an experimental setting.
Think of it like this: A chef meticulously follows a recipe, expecting a specific outcome. If one of the key ingredients is unknowingly tainted, the final dish will not only taste different but could also be inedible. Similarly, in research, if a peptide intended to bind to a specific receptor contains an impurity that also binds (or inhibits binding), your experimental observations will be misleading.
What Constitutes “Impurity” in Peptides?
Impurities in peptide synthesis can come in several forms:
- Truncated Sequences: Peptides that are shorter than the intended sequence due to incomplete coupling reactions.
- Deletion Sequences: Missing one or more amino acids within the sequence.
- Insertion Sequences: Extra amino acids incorporated into the sequence.
- Side Chain Modifications: Unwanted changes to the amino acid side chains during synthesis or handling.
- Racemization: Conversion of an L-amino acid to a D-amino acid, altering the peptide’s stereochemistry and potentially its activity.
- Residual Solvents and Reagents: Leftover chemicals from the synthesis process.
- Salts and Water: Though often less problematic, high concentrations can affect accurate weighing and formulation.
The presence of any of these contaminants, even in small amounts, can have significant implications for your research. For instance, a researcher studying the effects of BPC-157 on tissue repair would need absolute certainty that their BPC-157 sample is indeed BPC-157 and not a mixture of truncated peptides that might exhibit different, or even detrimental, effects. This is why testing peptide purity is an indispensable step.
“In the complex world of peptide research, purity isn’t a luxury, it’s the bedrock of credible scientific discovery. Without rigorous peptide purity testing, you’re building on sand.” 🔬
The Impact on Research Outcomes
Consider a scenario in 2025 where a pharmaceutical company is investing heavily in a novel peptide-based therapeutic. Early-stage research requires precise data to demonstrate efficacy and safety. If the initial research used peptides with, say, 85% purity instead of the desired 99%, the observed biological activity might be attributed to the intended peptide when, in reality, contaminants are influencing the results. This could lead to:
- False Positives/Negatives: Incorrectly concluding that a peptide has or lacks a certain effect.
- Inconsistent Results: Inability to reproduce findings across different experiments or labs.
- Increased Costs: Wasted reagents, time, and resources on experiments based on unreliable materials.
- Safety Concerns: In therapeutic applications, impurities could lead to adverse reactions.
- Delayed Progress: Setbacks in drug development or fundamental scientific understanding.
Therefore, ensuring 99% purity peptides through diligent peptide purity testing is not merely a best practice; it is an ethical imperative and a cornerstone of sound scientific methodology. This rigorous approach is particularly crucial when dealing with complex peptide blends like CJC-1295 Plus Ipamorelin or when exploring adaptive capacity and peptide mapping.
Understanding Peptide Purity Testing Methodologies
When you decide to send peptides for purity testing, it’s essential to understand the primary analytical techniques laboratories employ. These methods are designed to separate and identify components within a sample, providing a detailed profile of its composition. The most common and reliable methods for testing peptide purity are High-Performance Liquid Chromatography (HPLC) and Mass Spectrometry (MS).
High-Performance Liquid Chromatography (HPLC)
HPLC is considered the gold standard for peptide purity testing. It’s a powerful analytical technique used to separate, identify, and quantify each component in a mixture.
How it Works:
- Sample Injection: A small amount of the dissolved peptide sample is injected into the HPLC system.
- Separation Column: The sample is pushed by a liquid mobile phase through a column packed with a stationary phase (typically silica-based particles). The stationary phase has specific chemical properties (e.g., reverse-phase, where it’s hydrophobic).
- Differential Interaction: As the peptide and its impurities pass through the column, they interact differently with the stationary phase based on their chemical properties (e.g., hydrophobicity, charge, size).
- Elution and Detection: Components that interact less with the stationary phase move faster and elute first, while those that interact more are retained longer. As each component elutes from the column, it passes through a detector (commonly UV-Vis), which measures its absorbance.
- Chromatogram Generation: The detector generates a chromatogram – a graph plotting signal intensity against time. Each peak on the chromatogram represents a different compound. The area under each peak is proportional to the concentration of that compound.
Interpreting HPLC Results:
A typical HPLC chromatogram for a highly pure peptide will show one dominant, sharp peak, representing the target peptide, and ideally very small or no other peaks, which would indicate impurities. The purity is calculated as the area of the main peptide peak divided by the total area of all peaks in the chromatogram, expressed as a percentage. For example, if the main peak constitutes 99% of the total area, you have 99% purity peptides.
Advantages of HPLC for Peptide Purity Testing:
- High Resolution: Excellent separation capabilities for closely related compounds.
- Quantitative: Provides accurate percentages of each component.
- Versatile: Can be optimized for a wide range of peptide sizes and chemistries.
Limitations:
- Co-elution: Sometimes, two different impurities might elute at the same time, appearing as a single peak, which can slightly overestimate purity.
- Does Not Identify Structure: While it tells you how much of a component is present, it doesn’t definitively tell you what that component is. This is where Mass Spectrometry comes in.
Mass Spectrometry (MS)
Mass Spectrometry is often used in conjunction with HPLC (LC-MS) to provide definitive identification of the components separated by HPLC.
How it Works:
- Ionization: Peptides are first ionized (given an electrical charge) in a vacuum.
- Mass-to-Charge Ratio (m/z) Separation: The ionized peptides are then accelerated through an electric or magnetic field. Their flight path or time of flight is dependent on their mass-to-charge ratio (m/z).
- Detection: A detector measures the m/z ratio of each ion.
Interpreting MS Results:
The MS spectrum shows a series of peaks, each corresponding to a specific m/z ratio. By analyzing these peaks, researchers can determine the molecular weight of the peptide and its impurities. This is crucial for confirming the correct peptide sequence and identifying any truncated or modified forms. For example, if your target peptide has a theoretical molecular weight of 2000 Da, and MS shows a dominant peak at 2000 Da and smaller peaks at 1800 Da, the latter could indicate a deletion sequence.
Advantages of MS for Peptide Purity Testing:
- Structural Confirmation: Provides definitive molecular weight information, crucial for identifying what the impurities are.
- High Sensitivity: Can detect very low levels of impurities.
- Complementary to HPLC: When coupled (LC-MS), it offers both separation and identification capabilities, making it a powerful tool for thorough testing peptide purity.
Other Techniques
While HPLC and MS are the primary tools, other techniques may be used for specific analyses:
- Amino Acid Analysis (AAA): Confirms the amino acid composition of the peptide.
- Karl Fischer Titration: Measures water content.
- Endotoxin Testing: Crucial for peptides intended for in vivo studies, as endotoxins can elicit strong immune responses.
By utilizing these advanced analytical methods, reputable laboratories can provide a comprehensive assessment, ensuring the quality and integrity of your research materials, leading to confidence in your 99% purity peptides. Researchers often use these methods to verify the quality of peptides like Epithalon or to compare different peptide formulations.
The Certificate of Analysis (CoA): Your Quality Guarantee
When you send peptides for purity testing, the most important document you will receive back is the Certificate of Analysis (CoA). This document is your official confirmation of the peptide’s quality, purity, and identity. Understanding how to read and interpret a CoA is crucial for any researcher.
What to Expect on a CoA
A comprehensive CoA for a peptide should include the following information:
- Product Information:
- Peptide Name: The full name of the peptide (e.g., BPC-157).
- CAS Number: Chemical Abstracts Service registry number, a unique identifier.
- Lot Number: A specific identifier for the batch of peptide, allowing for traceability.
- Catalog Number: The supplier’s internal product code.
- Sequence: The amino acid sequence of the peptide.
- Molecular Weight: The calculated molecular weight.
- Analytical Results:
- HPLC Purity: This is typically the most prominent metric. It will state the percentage purity determined by HPLC. A high-quality CoA will show figures like >98% or >99% purity peptides.
- Mass Spectrometry (MS) Data: Often includes the observed molecular mass, which should match the theoretical molecular weight. A diagram or table of the MS results might also be included.
- Water Content: Measured by Karl Fischer titration, typically expressed as a percentage. Excessive water can dilute the peptide and affect its stability.
- Acetate/Counterion Content: Peptides are often synthesized as acetate salts. The percentage of acetate or other counterions will be listed.
- Amino Acid Analysis (AAA): May be included to confirm the amino acid composition.
- Endotoxin Level: Crucial for in vivo research, typically expressed in Endotoxin Units (EU) per milligram (e.g., < 1 EU/mg).
- Storage and Handling Recommendations:
- Specific instructions for storing the peptide (e.g., -20°C, desiccated) to maintain its stability.
- Recommendations for reconstitution and handling. (For more details, see Best Practices for Storing Research Peptides).
- Date of Analysis and Analyst Signature: Confirms when the testing was performed and by whom, adding to the document’s authenticity.
An Anecdote: The Case of the Missing Peak
Dr. Aris, a young biochemist in 2025, was excited to begin a new study on a promising peptide, 5-amino-1MQ. He purchased a batch online, and it arrived quickly. The accompanying CoA stated 95% purity by HPLC. Confident, he proceeded with his experiments. However, his initial results were inconsistent, defying established literature. Frustrated, he decided to send a sample for independent peptide purity testing at a third-party lab.
The new CoA came back, revealing a different story. While the main peak was indeed 5-amino-1MQ at 90% purity, there was a significant additional peak, representing 8% of the total area, which the accompanying MS analysis identified as a known inhibitor of the target pathway! The original CoA had either missed it or deliberately obscured it. Dr. Aris realized his initial “95% pure” peptide was, in effect, only 90% active material mixed with a potent contaminant. This experience underscored the absolute necessity of reliable testing peptide purity and the importance of critically evaluating every CoA, especially when purchasing research materials. This experience led him to look for suppliers who emphasize 5-amino-1mq peptides for sale with robust verification.
What Makes a Good CoA?
A reliable CoA is:
- Comprehensive: Contains all the details listed above.
- Transparent: Clearly shows the chromatograms and MS spectra, not just the numbers.
- Traceable: Links directly to a specific lot number.
- Recent: Analysis dates should be reasonably current, especially for more sensitive peptides.
- From a Reputable Source: Issued by an accredited laboratory with a proven track record.
Always scrutinize the CoA you receive. If anything looks incomplete, generic, or lacks detailed analytical data, it’s a red flag. For researchers committed to the highest standards, choosing suppliers like Pure Tested Peptides who provide transparent COAs and prioritize 99% purity peptides is non-negotiable.
Choosing a Reputable Laboratory for Peptide Purity Testing
The reliability of your peptide purity testing is directly tied to the competence and integrity of the laboratory you choose. In 2025, with many options available, discerning a truly reputable service provider is key.
Qualities of an Excellent Peptide Testing Lab
When selecting a lab to send your peptides for analysis, look for the following characteristics:
- Accreditation and Certification:
- ISO 17025 Accreditation: This international standard specifies the general requirements for the competence of testing and calibration laboratories. It signifies that the lab has a robust quality management system and is technically competent to produce precise and accurate test results. This is a crucial indicator of reliability for testing peptide purity.
- GMP (Good Manufacturing Practices) Compliance: While more focused on manufacturing, labs that adhere to GMP principles often have stricter quality control measures in place, which benefits analytical services.
- Expertise and Experience:
- Specialization in Peptides: Look for labs that specifically advertise expertise in peptide analysis, as peptides can be challenging to work with due to their diverse properties.
- Experienced Staff: Inquire about the experience and qualifications of their analytical chemists and technicians. They should be well-versed in HPLC, MS, and other relevant techniques.
- State-of-the-Art Equipment:
- The lab should possess modern, well-maintained analytical instrumentation, including high-resolution HPLC systems (e.g., UPLC for faster analysis), advanced mass spectrometers (e.g., Q-TOF, Orbitrap for higher accuracy), and other supporting equipment. Outdated equipment can lead to less precise or inaccurate results.
- Comprehensive Service Offerings:
- Beyond basic purity, can they perform amino acid analysis, water content, endotoxin testing, and counterion analysis? A full suite of services ensures you get a complete picture of your peptide’s quality.
- Transparent Reporting:
- A good lab will provide a detailed CoA, including raw chromatograms and MS spectra, not just summarized data. They should be willing to explain their results and answer any questions you have.
- Quick Turnaround Time (TAT) and Communication:
- While precision should not be sacrificed for speed, a reputable lab will offer reasonable TATs and keep you informed about the progress of your samples. Clear and timely communication is a sign of professionalism.
- Confidentiality and Data Security:
- Ensure the lab has robust policies in place to protect your intellectual property and experimental data.
Questions to Ask Potential Labs
Before committing to a lab, consider asking these questions:
- “Are you ISO 17025 accredited for peptide analysis?”
- “What specific HPLC and MS instruments do you use for peptide purity testing?”
- “Can you provide an example CoA for a peptide analysis, including raw data?”
- “What is your typical turnaround time for peptide purity analysis?”
- “What are your sample submission requirements (e.g., minimum quantity, required concentration)?”
- “How do you ensure sample integrity during testing?”
- “What quality control measures do you have in place?”
By thoroughly vetting potential laboratories, you can confidently send peptides for purity testing, knowing that you will receive accurate and reliable results that stand up to scientific scrutiny. This diligent approach is critical, whether you’re researching 5-amino-1MQ or exploring complex peptide blends for research.
Preparing and Sending Your Peptide Samples for Purity Testing

Proper sample preparation and shipping are just as important as the analytical methods themselves. Mishandling can degrade your peptide, leading to inaccurate purity readings or even rendering your sample unusable. Here’s a step-by-step guide to ensure your samples arrive at the lab in optimal condition.
Step 1: Handling and Storage Prior to Shipping
- Maintain Cold Chain: Most peptides are sensitive to heat and light. Keep your peptide samples stored according to the manufacturer’s recommendations (typically -20°C or colder, desiccated) until just before packaging. Avoid repeated freeze-thaw cycles.
- Minimize Exposure: When handling, work quickly and in a clean environment. Use sterile tools. Peptides can be susceptible to degradation from moisture, oxygen, and certain plastics.
- Record Keeping: Label your vials clearly with the peptide name, lot number, date, and your internal reference number. Maintain a detailed log of your peptide inventory.
Step 2: Sample Preparation for Shipment
- Choose Appropriate Vials: Use clean, dry, screw-cap vials (e.g., borosilicate glass vials with PTFE-lined caps) that are certified for laboratory use. Avoid plastic vials if there’s a risk of peptide adsorption or leaching of plasticizers.
- Quantity: Confirm the minimum quantity required by the testing laboratory. Typically, a few milligrams (e.g., 2-5 mg) is sufficient for initial purity testing.
- Formulation: Peptides are often shipped as lyophilized (freeze-dried) powders, which are generally more stable. If your peptide is in solution, consider lyophilizing it before shipment if the lab recommends it, or discuss appropriate liquid shipping protocols. If shipping in solution, ensure the solvent is compatible and stable, and the concentration is known.
Step 3: Packaging for Shipment
The goal is to protect the peptide from physical damage, temperature fluctuations, and moisture during transit.
- Primary Container: Place each peptide vial into a secondary, sealed container (e.g., a small plastic zip-lock bag) to contain any leakage and protect it from condensation.
- Absorbent Material: Include absorbent material (e.g., paper towels, cotton wool) within the secondary container.
- Insulated Container: Place the sealed secondary containers into an insulated shipping box or Styrofoam cooler.
- Cold Packs/Dry Ice:
- For Refrigerated Peptides: Use gel packs or frozen ice packs. Ensure they are completely frozen before packing.
- For Frozen Peptides (especially sensitive ones): Use dry ice. Pack enough dry ice to last for at least 24-48 hours longer than the estimated transit time, as dry ice sublimates. Wear appropriate PPE (gloves, eye protection) when handling dry ice, and ensure the shipping container is vented to prevent pressure buildup.
- Cushioning Material: Fill any empty space in the insulated container with cushioning material (e.g., bubble wrap, foam peanuts) to prevent vials from shifting and breaking.
- Documentation: Include all necessary documentation inside a separate waterproof bag within the shipping box, including:
- A packing list: Itemizing each peptide sample.
- Your contact information.
- The laboratory’s submission form: Often provided by the lab, detailing the tests requested for each sample.
Step 4: Shipping Logistics
- Choose a Reliable Carrier: Use reputable courier services known for handling temperature-sensitive biological samples (e.g., FedEx, UPS, DHL).
- Expedited Shipping: Always opt for overnight or expedited shipping to minimize transit time and temperature exposure.
- Tracking: Ensure you get a tracking number and monitor the shipment’s progress.
- Customs Declarations (International Shipments): For international shipments, declare the contents accurately (e.g., “Research Samples – Non-hazardous – Non-infectious”) to avoid delays. Provide proper documentation for customs.
Pro Tip: “Always communicate with the receiving laboratory before shipping your samples. They can provide specific instructions, submission forms, and best practices tailored to their facility.”
By following these meticulous steps, you significantly increase the chances of your peptide samples arriving at the testing facility intact and ready for accurate peptide purity testing, ensuring that the results reflect the true quality of your 99% purity peptides. This level of care is essential for any serious research, whether you are dealing with common peptides or researching complex interactions like the synergy of LL37 and mots-c.
Interpreting Your Peptide Purity Results and Taking Action
Once your samples have been analyzed, and you receive the Certificate of Analysis (CoA), the next crucial step is to correctly interpret the data and decide on your next actions. Understanding these results is vital for maintaining the integrity of your research in 2025.
What Does the Purity Percentage Really Mean?
As discussed, the HPLC purity percentage indicates the proportion of the main peptide peak relative to all other detected peaks.
- 99%+ Purity: This is the ideal scenario for 99% purity peptides. Such samples are considered extremely high quality and suitable for virtually any research application, including highly sensitive in vivo studies. You can proceed with your experiments with high confidence.
- 95-98% Purity: These peptides are still considered very good quality. For many in vitro applications or initial screening studies, this level of purity may be acceptable. However, for critical dose-response studies or in vivo work, it’s essential to consider the nature of the impurities. Are they related sequences that might have some activity, or are they inert? The MS data becomes critical here.
- Below 95% Purity: For most serious research, a peptide with purity below 95% should be viewed with caution. The presence of significant impurities (5% or more) can confound results, making it difficult to attribute observed effects solely to the intended peptide.
- Action: If your peptide falls into this category, you have a few options:
- Discuss with the Lab: Get detailed information on the identified impurities from the MS data.
- Repurification: Some specialized labs can offer custom repurification services if the impurity profile is amenable. However, this can be costly.
- Re-order from a Different Supplier: If the cost of repurification is prohibitive or the impurities are too complex, it might be more economical and reliable to source new peptide from a different, more reputable supplier that guarantees 99% purity peptides.
- Action: If your peptide falls into this category, you have a few options:
Considering Related Impurities
The quality of the MS data is paramount when dealing with purities below 99%. Sometimes, the “impurities” detected by HPLC are closely related peptide fragments (e.g., a one-amino-acid deletion) that might still exhibit some biological activity, albeit altered. In other cases, the impurities might be entirely unrelated synthetic byproducts or even degradation products.
- Example: If you’re researching AOD-9604, and the CoA shows 96% purity with a 3% impurity identified as a very similar AOD-9604 analog, your experimental results might still be interpretable, but with a note of caution regarding potential cross-reactivity or altered potency. However, if the 3% impurity is a completely unrelated compound, your confidence in the results would be significantly lower.
Beyond Purity: Other CoA Factors
Remember to look at other critical parameters on your CoA:
- Water Content: High water content means you’re paying for water, not peptide. It also reduces the stability of lyophilized peptides. Factor this into your calculations for reconstituting the peptide.
- Counterion Content: The counterion (e.g., acetate, TFA) can also contribute to the “non-peptide” mass. While generally less concerning than peptide impurities, it’s good to be aware of the actual peptide content.
- Endotoxin Levels: For in vivo studies, even highly pure peptides must be low in endotoxins. If the endotoxin level is too high, the peptide is unsuitable for animal or cell culture studies unless specifically treated.
Actionable Next Steps
- Document and Cross-Reference: File your CoA with your peptide inventory. Cross-reference the lot number with your experimental records.
- Adjust Concentrations: If the purity is less than 100%, adjust your working stock concentrations accordingly to ensure you are adding the precise amount of the active peptide. For example, if you aim for 1 µM and your peptide is 95% pure, you’ll need to add slightly more of the raw material to achieve the true 1 µM active peptide concentration.
- Source Wisely for Future: If you received unsatisfactory results, re-evaluate your peptide supplier. Look for vendors who explicitly state they provide 99% purity peptides and back it up with transparent, third-party COAs. Many researchers depend on verified sources for compounds like CJC-1295 without DAC.
- Consider Repurification: For very specific, valuable peptides with known but manageable impurities, consider sending them for repurification if the cost-benefit analysis makes sense for your research.
- Ethical Considerations: If you suspect a supplier is providing misleading purity data, consider reporting it to relevant scientific bodies or reviewing platforms to protect other researchers.
By diligently interpreting your peptide purity testing results and taking appropriate action, you uphold the scientific rigor of your research and ensure that your findings are reliable and reproducible. This commitment to quality is what drives true innovation in 2025 and beyond.
Conclusion
In the competitive and rigorous landscape of scientific research in 2025, the pursuit of precision and reliability is paramount. For anyone working with peptides, the quality of your materials directly translates to the integrity and reproducibility of your experimental outcomes. Sending peptides for purity testing is not merely an optional step; it is a fundamental requirement for any researcher dedicated to generating credible, publishable results.
We’ve explored why peptide purity testing is so critical, delving into the nuances of impurities and their potential to derail promising studies. We’ve demystified the analytical powerhouse duo of HPLC and Mass Spectrometry, the backbone of accurate testing peptide purity, and dissected the Certificate of Analysis (CoA) as your ultimate assurance of quality. Furthermore, we’ve provided a comprehensive guide to selecting a reputable laboratory and meticulously preparing your precious samples for shipment, ensuring they arrive ready for analysis. Finally, we’ve walked through the crucial process of interpreting your purity results and outlined actionable steps to safeguard your research.
The investment in ensuring 99% purity peptides through rigorous testing is an investment in your research, your reputation, and the advancement of science itself. Don’t let unforeseen impurities compromise your hard work. Prioritize quality, demand transparency, and always verify your peptide samples.
Actionable Next Steps for Researchers:
- Audit Your Suppliers: Review your current peptide suppliers. Do they provide comprehensive, verifiable COAs for their products? If not, consider switching to providers known for their commitment to quality and transparency, such as those found on Pure Tested Peptides.
- Plan for Testing: Incorporate third-party peptide purity testing into your research workflow, especially for critical experiments or when sourcing from new suppliers. Budget for this essential quality control step.
- Educate Your Team: Ensure everyone in your lab understands the importance of peptide purity, proper handling, and the interpretation of COAs.
- Stay Informed: Keep abreast of advancements in peptide synthesis and analytical techniques. The field is constantly evolving, and staying informed will help you make the best decisions for your research.
By embracing these practices, you can confidently navigate the complexities of peptide research in 2025, knowing that your findings are built upon the solid foundation of high-quality, rigorously tested materials. Your commitment to precision will undoubtedly accelerate discovery and contribute meaningfully to scientific progress.
References
[1] Hancock, W. S. (2002). Peptide Analysis. eLS.
[2] Kinter, M., & Sherman, N. E. (2000). Protein Sequencing and Identification Using Tandem Mass Spectrometry. John Wiley & Sons.
[3] European Pharmacopoeia 10.0 (2020). 5.1.10. Guidelines for the quality of non-compendial products. EDQM.
SEO Meta Title: Peptide Purity Testing: Ensure 99% Pure Peptides in 2025
SEO Meta Description: Learn to send peptides for purity testing in 2025. Discover HPLC/MS methods, interpret COAs, and ensure 99% purity peptides for reliable research results.