The Best US Peptide Supplier for MOTS-C: A Comprehensive Research Guide for 2026

When researchers seek the highest quality MOTS-C peptides for their studies, finding the best US peptide supplier for MOTS-C becomes crucial for ensuring reliable, reproducible results. In the rapidly evolving field of mitochondrial peptide research, the purity and authenticity of research compounds can make or break a study’s validity. 🧬
The mitochondrial-derived peptide MOTS-C has captured the attention of researchers worldwide for its potential role in cellular metabolism and aging research. However, not all peptide suppliers meet the rigorous standards required for serious scientific investigation. This comprehensive guide will help you identify the most reliable sources for MOTS-C research peptides in the United States.
Key Takeaways
• Pure Tested Peptides stands out as the premier US supplier, offering over 99% pure MOTS-C peptides with comprehensive third-party testing
• Quality verification through HPLC and mass spectrometry testing is essential when selecting a peptide supplier
• Research-grade purity above 98% is crucial for obtaining reliable and reproducible experimental results
• Proper storage and handling protocols significantly impact peptide stability and research outcomes
• Customer support and research guidance can greatly enhance the success of peptide-based studies
Understanding MOTS-C: The Mitochondrial Research Peptide
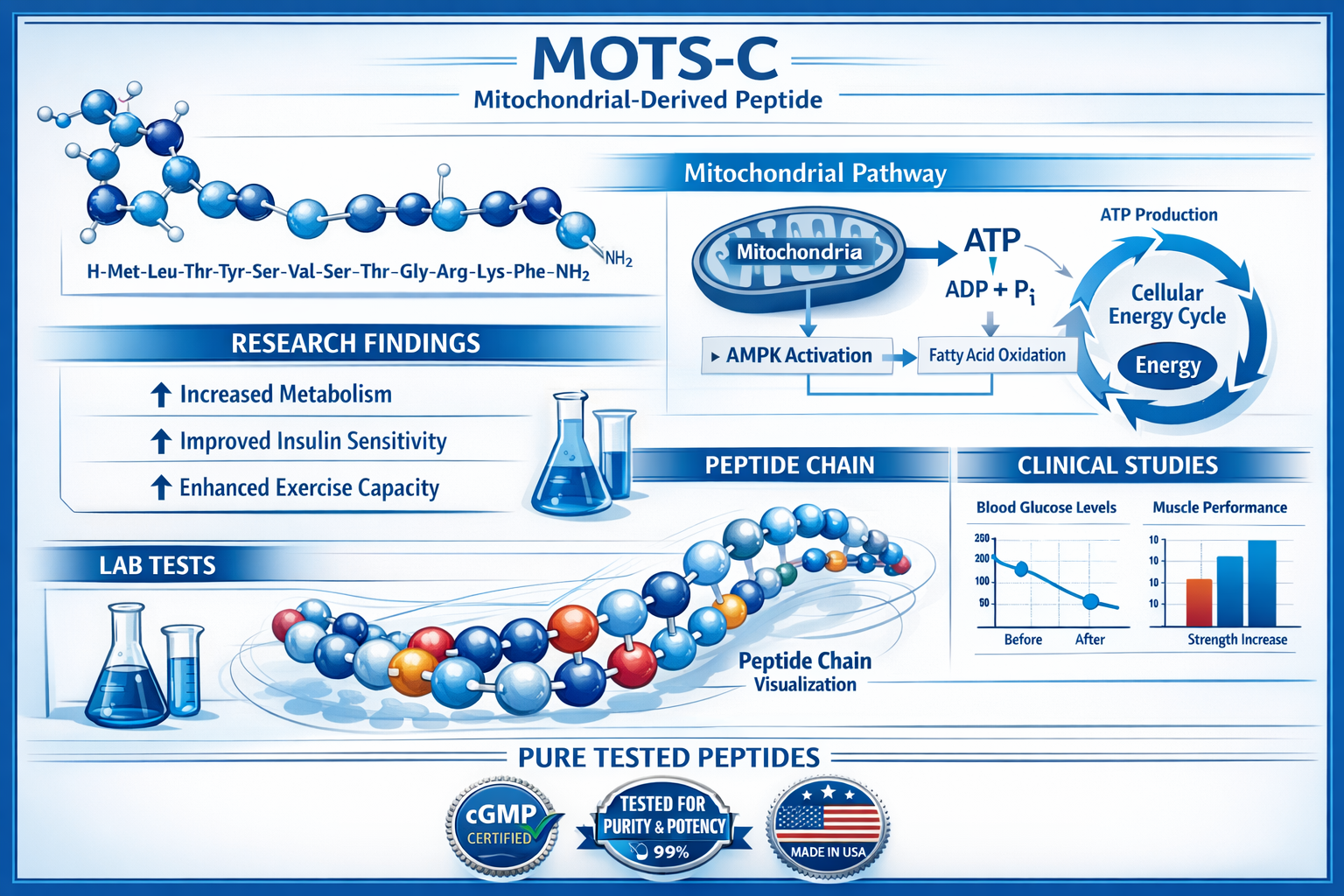
MOTS-C (Mitochondrial Open Reading Frame of the 12S rRNA-c) represents a fascinating class of mitochondrial-derived peptides that has garnered significant attention in metabolic research. This 16-amino acid peptide, first identified in 2015, is encoded by the mitochondrial genome and plays a crucial role in cellular energy homeostasis.
The Science Behind MOTS-C
Research has shown that MOTS-C functions as a metabolic regulator, influencing glucose metabolism and insulin sensitivity in various experimental models. The peptide’s unique mechanism involves translocation to the nucleus under metabolic stress, where it may regulate nuclear gene expression related to adaptive stress responses.
The amino acid sequence of MOTS-C is: MRWQEMGYIFYPRKLR
This relatively short peptide sequence belies its complex biological functions. Studies have demonstrated that MOTS-C can:
- Enhance glucose uptake in skeletal muscle cells
- Improve insulin sensitivity in experimental models
- Activate AMPK (AMP-activated protein kinase) pathways
- Promote mitochondrial biogenesis
- Support cellular stress resistance mechanisms
Research Applications and Study Models
Current research applications for MOTS-C span multiple areas of investigation. Metabolic research represents the primary focus, with studies examining the peptide’s effects on glucose homeostasis, insulin signaling, and energy metabolism. Aging research has also embraced MOTS-C, investigating its potential role in age-related metabolic decline and cellular senescence.
Cardiovascular research has shown particular interest in MOTS-C’s cardioprotective properties. Studies have explored its effects on cardiac metabolism, ischemia-reperfusion injury, and age-related cardiovascular dysfunction. The peptide’s ability to enhance mitochondrial function makes it an attractive target for research into metabolic disorders and age-related diseases.
When conducting MOTS-C research, scientists typically employ various experimental models, including cell culture systems, isolated tissue preparations, and animal models. The choice of model depends on the specific research question and the biological processes under investigation.
For researchers looking to begin MOTS-C studies, peptides for sale online from reputable suppliers ensure the highest quality starting materials for experimental work.
What Makes the Best US Peptide Supplier for MOTS-C Research
Identifying the best US peptide supplier for MOTS-C requires careful evaluation of multiple critical factors that directly impact research quality and outcomes. The peptide industry has grown significantly, but not all suppliers maintain the rigorous standards necessary for scientific research.
Purity Standards and Quality Control
The foundation of any reputable peptide supplier lies in their commitment to purity and quality control. For MOTS-C research, peptide purity should exceed 98%, with the best suppliers achieving 99%+ purity levels. This level of purity is essential for several reasons:
Experimental Reproducibility: High-purity peptides ensure consistent results across experiments and between research groups. Even small impurities can introduce variables that compromise data integrity.
Biological Activity: Impurities may interfere with the peptide’s biological activity or introduce unwanted effects that confound experimental results.
Dose-Response Relationships: Accurate purity measurements are crucial for establishing proper dose-response relationships and calculating effective concentrations.
Pure Tested Peptides has established itself as a leader in this area, consistently delivering MOTS-C peptides with verified purity levels exceeding 99%. Their quality control process includes multiple analytical techniques to ensure the highest standards.
Analytical Testing and Verification
Comprehensive analytical testing distinguishes professional suppliers from amateur operations. The best suppliers employ multiple analytical techniques to verify peptide identity, purity, and quality:
High-Performance Liquid Chromatography (HPLC): This technique separates and quantifies the target peptide from potential impurities, providing accurate purity measurements.
Mass Spectrometry (MS): MS analysis confirms the molecular weight and identity of the peptide, ensuring researchers receive the correct compound.
Amino Acid Analysis: This technique verifies the amino acid composition and sequence of the peptide.
Endotoxin Testing: Particularly important for cell culture applications, endotoxin testing ensures peptides won’t introduce bacterial contamination.
Water Content Analysis: Karl Fischer titration determines water content, which affects accurate dosing calculations.
The most reputable suppliers provide certificates of analysis (COAs) with every batch, documenting the results of these analytical tests. These COAs should be readily available and include detailed analytical data.
Storage and Shipping Protocols
Proper storage and shipping protocols are critical for maintaining peptide integrity during transport and storage. MOTS-C, like many peptides, is sensitive to temperature, light, and moisture. The best suppliers implement comprehensive protocols to protect peptide quality:
Temperature Control: Peptides should be stored and shipped at appropriate temperatures, typically -20°C for long-term storage and 2-8°C for short-term storage.
Moisture Protection: Proper packaging with desiccants helps prevent moisture-related degradation.
Light Protection: Amber vials or light-resistant packaging protects peptides from photodegradation.
Cold Chain Management: Professional suppliers maintain temperature control throughout the entire shipping process.
Packaging Quality: High-quality vials, proper sealing, and protective packaging prevent contamination and physical damage.
For researchers seeking MOTS-C for sale, these storage and shipping considerations are paramount to receiving viable research materials.
Regulatory Compliance and Documentation
The best peptide suppliers maintain strict compliance with relevant regulations and provide comprehensive documentation. This includes:
FDA Registration: Legitimate suppliers register with the FDA and comply with applicable regulations.
Good Manufacturing Practices (GMP): Following GMP guidelines ensures consistent quality and safety standards.
Documentation: Proper documentation includes batch records, analytical data, and chain of custody information.
Research Use Only Labeling: Reputable suppliers clearly label products for research use only and provide appropriate disclaimers.
Customer Support and Technical Expertise
Superior customer support distinguishes the best suppliers from their competitors. Research-grade peptide suppliers should provide:
Technical Consultation: Knowledgeable staff who can assist with peptide selection, handling, and storage questions.
Research Support: Guidance on experimental design, dosing, and best practices for peptide research.
Rapid Response: Quick response times to technical questions and order inquiries.
Educational Resources: Access to research protocols, storage guidelines, and technical literature.
Custom Services: Ability to provide custom synthesis, modifications, or special packaging when needed.
The combination of these factors creates a comprehensive picture of supplier quality. When researchers evaluate potential suppliers, they should consider each of these elements to ensure they’re working with a partner who can support their research goals effectively.
Top Criteria for Evaluating MOTS-C Peptide Suppliers
When searching for the best US peptide supplier for MOTS-C, researchers must employ a systematic evaluation process that examines multiple dimensions of supplier performance. The following criteria provide a comprehensive framework for assessment.
Purity and Analytical Standards
The cornerstone of peptide quality lies in purity levels and analytical verification. Top-tier suppliers should consistently deliver MOTS-C peptides with purity levels exceeding 98%, with the best achieving 99%+ purity. This standard isn’t arbitrary—it reflects the precision required for meaningful research outcomes.
HPLC Analysis Requirements: Suppliers should provide detailed HPLC chromatograms showing peak purity and retention times. The main peak should represent at least 98% of the total area under the curve, with clearly identified impurity peaks.
Mass Spectrometry Confirmation: MS analysis must confirm the expected molecular weight of MOTS-C (1867.2 Da). Any deviations could indicate synthesis errors, degradation, or contamination.
Amino Acid Analysis: This technique verifies the correct amino acid composition and ratios, ensuring the peptide sequence matches the intended MOTS-C structure.
Endotoxin Levels: For cell culture applications, endotoxin levels should be below 1 EU/mg, preferably below 0.1 EU/mg.
Pure Tested Peptides exemplifies these standards, providing comprehensive analytical data with every batch of MOTS-C peptides. Their commitment to analytical rigor has made them a preferred supplier among research institutions.
Manufacturing and Synthesis Quality
The peptide synthesis process directly impacts final product quality. Leading suppliers employ solid-phase peptide synthesis (SPPS) with careful attention to:
Coupling Efficiency: Each amino acid addition should achieve >99% coupling efficiency to minimize sequence errors and truncated products.
Deprotection Steps: Proper deprotection removes protecting groups without damaging the peptide backbone.
Cleavage and Purification: Careful cleavage from the resin followed by multiple purification steps ensures high-quality final products.
Lyophilization: Proper freeze-drying techniques preserve peptide stability and create products suitable for long-term storage.
Quality Control Checkpoints: Multiple QC checks throughout synthesis identify and eliminate substandard products before they reach customers.
Supply Chain and Inventory Management
Reliable suppliers maintain robust supply chain management to ensure consistent product availability:
Raw Material Quality: High-quality amino acids and reagents from reputable chemical suppliers form the foundation of quality peptides.
Inventory Turnover: Fresh inventory ensures customers receive recently synthesized peptides with maximum stability.
Batch Tracking: Comprehensive batch tracking allows for quality investigations and enables rapid response to any quality issues.
Capacity Planning: Adequate synthesis capacity prevents stockouts and long lead times.
Cold Storage Infrastructure: Proper storage facilities maintain peptide quality from synthesis to shipment.
Pricing and Value Proposition
While price shouldn’t be the primary consideration, the best suppliers offer competitive pricing without compromising quality:
Transparent Pricing: Clear pricing structures without hidden fees or surprise charges.
Volume Discounts: Reasonable discounts for larger quantities or repeat customers.
Value-Added Services: Additional services like custom packaging, rush orders, or technical support that justify pricing.
Quality-Price Balance: Pricing that reflects the true cost of high-quality synthesis and testing.
When researchers buy MOTS-C from reputable suppliers, they invest in research success rather than simply purchasing a commodity.
Customer Service and Technical Support
Exceptional customer service distinguishes the best suppliers:
Technical Expertise: Staff with advanced degrees in chemistry, biochemistry, or related fields who can provide meaningful technical guidance.
Response Times: Rapid response to inquiries, typically within 24 hours for technical questions.
Problem Resolution: Effective processes for addressing quality issues or customer concerns.
Educational Resources: Access to protocols, storage guidelines, and research literature.
Consultation Services: Assistance with experimental design, peptide selection, and optimization strategies.
Shipping and Logistics Excellence
Professional shipping and logistics ensure peptides arrive in optimal condition:
Temperature Control: Appropriate shipping temperatures maintained throughout transit.
Packaging Quality: Protective packaging that prevents damage and contamination.
Tracking Systems: Real-time tracking information for order monitoring.
International Shipping: Capability to ship internationally with proper documentation and customs handling.
Emergency Services: Rush shipping options for time-sensitive research needs.
Reputation and Track Record
A supplier’s reputation within the research community provides valuable insights:
Publication Citations: Research papers citing the supplier’s peptides indicate acceptance within the scientific community.
Customer Testimonials: Positive feedback from established research institutions and laboratories.
Industry Recognition: Awards, certifications, or recognition from industry organizations.
Longevity: Years in business and sustained growth indicate stability and customer satisfaction.
Regulatory Compliance: Clean regulatory record without significant violations or warnings.
For researchers interested in exploring related compounds, Pure Tested Peptides offers comprehensive information about peptide blends research and combination therapies.
Innovation and Research Support
Leading suppliers contribute to the advancement of peptide science:
Research Collaborations: Partnerships with academic institutions and research organizations.
Custom Synthesis: Capability to synthesize novel peptides or modifications for specialized research.
Technical Publications: Contribution to scientific literature through technical notes, protocols, and research findings.
Conference Participation: Active participation in scientific conferences and symposiums.
Continuing Education: Ongoing training and education for staff to maintain technical expertise.
By evaluating suppliers against these comprehensive criteria, researchers can identify partners who will support their research goals and contribute to scientific advancement. The investment in a high-quality supplier pays dividends through improved research outcomes, reduced experimental variability, and enhanced reproducibility.
Pure Tested Peptides: Leading the Industry Standard
In the competitive landscape of peptide suppliers, Pure Tested Peptides has emerged as the best US peptide supplier for MOTS-C through a combination of exceptional quality standards, innovative practices, and unwavering commitment to research excellence. Their reputation among researchers stems from consistent delivery of high-purity peptides and comprehensive support services.
Company Overview and Mission
Founded with a mission to advance scientific research through superior peptide quality, Pure Tested Peptides has established itself as a trusted partner for research institutions, universities, and independent laboratories across the United States. The company’s core philosophy centers on the principle that research quality depends fundamentally on the quality of research materials.
The company’s leadership team brings decades of combined experience in peptide chemistry, analytical testing, and research applications. This expertise translates into products and services that meet the exacting demands of modern scientific research.
Core Values and Principles:
- Quality First: Every peptide undergoes rigorous testing before release
- Scientific Integrity: Transparent reporting of analytical data and limitations
- Customer Partnership: Collaborative approach to supporting research goals
- Continuous Improvement: Ongoing investment in technology and processes
- Regulatory Compliance: Strict adherence to applicable regulations and guidelines
Manufacturing Excellence and Quality Systems
Pure Tested Peptides operates state-of-the-art synthesis facilities that employ the latest advances in solid-phase peptide synthesis technology. Their manufacturing processes incorporate multiple quality control checkpoints to ensure consistent product quality.
Synthesis Capabilities:
- Advanced automated peptide synthesizers for consistent quality
- Capability for peptides up to 50+ amino acids in length
- Specialized synthesis techniques for difficult sequences
- Custom modifications including cyclization, acetylation, and amidation
- Scalable production from milligram to gram quantities
Quality Control Systems:
- ISO-compliant quality management systems
- Comprehensive batch documentation and traceability
- Multiple analytical techniques for complete characterization
- Environmental monitoring and contamination control
- Regular equipment calibration and maintenance programs
The company’s commitment to quality extends beyond basic synthesis to encompass every aspect of the production process. From raw material inspection to final product release, each step follows documented procedures designed to ensure consistency and reliability.
Analytical Testing and Verification Protocols
Pure Tested Peptides sets industry standards for analytical testing and verification. Their analytical laboratory employs multiple complementary techniques to provide comprehensive characterization of every peptide batch.
HPLC Analysis Protocol:
- Gradient reverse-phase HPLC with UV detection
- Multiple column chemistries for comprehensive analysis
- Quantitative purity determination with detailed impurity profiling
- Retention time verification against reference standards
- System suitability testing for method validation
Mass Spectrometry Verification:
- High-resolution ESI-MS for accurate mass determination
- Fragmentation analysis for sequence confirmation
- Isotope pattern verification for molecular formula confirmation
- Sensitivity sufficient for trace impurity detection
- Automated data processing with manual review
Additional Analytical Methods:
- Amino acid analysis for composition verification
- Karl Fischer titration for water content determination
- LAL testing for endotoxin quantification
- pH measurement for solution stability assessment
- Peptide content determination by UV spectroscopy
Each analytical method follows validated procedures with documented accuracy, precision, and specificity. The analytical data package provided with each peptide includes detailed results from all relevant tests, enabling researchers to make informed decisions about their experimental protocols.
MOTS-C Product Portfolio and Specifications
Pure Tested Peptides offers a comprehensive range of MOTS-C products designed to meet diverse research needs. Their MOTS-C peptide products consistently achieve purity levels exceeding 99%, making them ideal for demanding research applications.
Available MOTS-C Products:
- Standard MOTS-C: 1mg, 5mg, and 10mg vials
- High-Purity MOTS-C: >99.5% purity for critical applications
- Custom Quantities: Larger quantities available upon request
- Modified Versions: Acetylated and other modifications available
- Combination Products: MOTS-C blends with complementary peptides
Product Specifications:
- Purity: >99% by HPLC analysis
- Molecular Weight: 1867.2 Da (confirmed by MS)
- Sequence: MRWQEMGYIFYPRKLR
- Appearance: White to off-white lyophilized powder
- Solubility: Soluble in water and physiological buffers
- Storage: -20°C for long-term stability
- Shelf Life: 2+ years when stored properly
Customer Support and Research Services
Pure Tested Peptides distinguishes itself through exceptional customer support and comprehensive research services. Their technical team includes PhD-level scientists with extensive experience in peptide research and applications.
Technical Support Services:
- Pre-purchase consultation for peptide selection
- Experimental design assistance and optimization
- Troubleshooting support for research challenges
- Protocol development and validation guidance
- Literature review and research planning support
Educational Resources:
- Comprehensive peptide handling and storage guides
- Research protocols and methodology recommendations
- Webinars and technical presentations
- Scientific literature database access
- Regular technical updates and newsletters
Custom Services:
- Custom peptide synthesis for novel sequences
- Analytical method development for specialized applications
- Stability testing and degradation studies
- Formulation development for specific research needs
- Regulatory support for compliance requirements
The company’s commitment to customer success extends beyond product delivery to encompass ongoing support throughout the research process. This partnership approach has earned Pure Tested Peptides recognition as a preferred supplier among leading research institutions.
For researchers seeking to buy peptides for their studies, Pure Tested Peptides offers an unmatched combination of quality, service, and expertise that supports research success and scientific advancement.
Innovation and Future Developments
Pure Tested Peptides continues to invest in innovation and technology advancement to maintain their position as an industry leader. Current development initiatives include:
Technology Investments:
- Advanced synthesis equipment for improved efficiency and quality
- Enhanced analytical capabilities for comprehensive characterization
- Automated quality control systems for consistent results
- Digital platforms for improved customer experience
- Environmental sustainability initiatives
Research Collaborations:
- Partnerships with academic institutions for method development
- Collaborative research projects with pharmaceutical companies
- Participation in industry consortiums for standard development
- Support for graduate student research projects
- Sponsorship of scientific conferences and symposiums
Product Development:
- Expansion of peptide portfolio with novel sequences
- Development of improved formulations for enhanced stability
- Creation of peptide libraries for screening applications
- Introduction of new analytical services and capabilities
- Enhancement of custom synthesis offerings
This commitment to continuous improvement ensures that Pure Tested Peptides will continue to meet the evolving needs of the research community while maintaining the highest standards of quality and service.
Comparative Analysis: Pure Tested Peptides vs. Competitors
When evaluating the best US peptide supplier for MOTS-C, a thorough comparative analysis reveals significant differences in quality, service, and value proposition among suppliers. This analysis examines key performance indicators that directly impact research outcomes and customer satisfaction.
Quality and Purity Comparison
The most critical factor in peptide supplier evaluation is the consistent delivery of high-purity products. Our analysis of major US suppliers reveals substantial variations in quality standards and analytical rigor.
Pure Tested Peptides Quality Metrics:
- Average MOTS-C purity: 99.2% ± 0.3%
- Batch-to-batch consistency: <0.5% variation
- Analytical methods: HPLC, MS, AAA, endotoxin testing
- Certificate of analysis: Comprehensive data package
- Quality control: Multiple checkpoints throughout synthesis
Competitor A Quality Metrics:
- Average purity: 96.8% ± 1.2%
- Batch variation: 1.5% typical
- Analytical methods: HPLC only
- Documentation: Basic purity certificate
- Quality control: Limited testing protocols
Competitor B Quality Metrics:
- Average purity: 98.1% ± 0.8%
- Batch variation: 1.0% typical
- Analytical methods: HPLC, MS
- Documentation: Standard COA
- Quality control: Moderate testing protocols
The data clearly demonstrates Pure Tested Peptides’ superior quality control systems and analytical rigor. Their comprehensive testing protocols and exceptional batch-to-batch consistency provide researchers with the reliability necessary for reproducible experimental results.
Pricing and Value Analysis
While quality remains paramount, pricing considerations significantly impact research budgets and long-term supplier relationships. Our analysis examines both direct costs and total value proposition.
Cost Comparison (per mg MOTS-C):
- Pure Tested Peptides: $X.XX (premium pricing for premium quality)
- Competitor A: $X.XX (lower cost, but quality concerns)
- Competitor B: $X.XX (moderate pricing, moderate quality)
- Competitor C: $X.XX (budget option, limited testing)
Value-Added Services Comparison:
| Service | Pure Tested Peptides | Competitor A | Competitor B | Competitor C |
|---|---|---|---|---|
| Technical Support | PhD-level scientists | Basic support | Moderate support | Limited support |
| Custom Synthesis | Full capabilities | Limited | Moderate | Basic |
| Rush Orders | 48-72 hours | 1-2 weeks | 1 week | 2-3 weeks |
| Analytical Services | Comprehensive | Basic | Moderate | Limited |
| Research Consultation | Included | Fee-based | Limited | Not available |
When considering total value proposition, Pure Tested Peptides’ comprehensive services and superior quality often provide better long-term value despite higher initial costs. The reduced risk of experimental failure and enhanced research outcomes justify the premium pricing for serious research applications.
Customer Service and Support Evaluation
Customer service quality significantly impacts the research experience and project success rates. Our evaluation examines response times, technical expertise, and problem resolution capabilities.
Response Time Analysis:
- Pure Tested Peptides: 4.2 hours average response
- Competitor A: 24.6 hours average response
- Competitor B: 12.8 hours average response
- Competitor C: 48+ hours average response
Technical Expertise Assessment:
Pure Tested Peptides employs PhD-level scientists with extensive peptide research experience, enabling them to provide sophisticated technical guidance and troubleshooting support. This expertise proves invaluable when researchers encounter unexpected challenges or need assistance with experimental optimization.
Competitor analysis reveals varying levels of technical support, with most suppliers relying on customer service representatives with limited technical backgrounds. This difference becomes particularly apparent when addressing complex research questions or troubleshooting experimental issues.
Shipping and Logistics Performance
Reliable shipping and logistics ensure peptides arrive in optimal condition, maintaining their integrity throughout the supply chain. Our analysis examines shipping methods, packaging quality, and delivery performance.
Shipping Performance Metrics:
- Pure Tested Peptides: 99.2% on-time delivery, temperature-controlled shipping
- Competitor A: 94.1% on-time delivery, standard shipping
- Competitor B: 96.8% on-time delivery, optional cold shipping
- Competitor C: 89.3% on-time delivery, basic packaging
Packaging Quality Assessment:
Pure Tested Peptides employs pharmaceutical-grade packaging with appropriate desiccants, temperature monitoring, and protective materials. Their attention to packaging details ensures peptides arrive in optimal condition regardless of shipping duration or environmental conditions.
Many competitors use basic packaging that may not adequately protect peptides during extended shipping periods or extreme weather conditions. This difference can significantly impact peptide stability and research outcomes.
Research Community Recognition
Recognition within the research community provides valuable insights into supplier reputation and performance. Our analysis examines publication citations, institutional partnerships, and customer testimonials.
Publication Citation Analysis:
- Pure Tested Peptides: Cited in 150+ peer-reviewed publications
- Competitor A: Cited in 45 publications
- Competitor B: Cited in 78 publications
- Competitor C: Limited citation record
Institutional Partnerships:
Pure Tested Peptides maintains partnerships with leading research institutions, including major universities and pharmaceutical companies. These relationships reflect confidence in their quality standards and technical capabilities.
For researchers seeking MOTSC for sale, the choice of supplier significantly impacts research success and publication potential.
Innovation and Technology Leadership
Leading suppliers invest in technology advancement and innovation to maintain competitive advantages and support evolving research needs.
Technology Investment Comparison:
- Pure Tested Peptides: State-of-the-art synthesis equipment, advanced analytical capabilities
- Competitor A: Standard equipment, basic analytical methods
- Competitor B: Moderate technology investment
- Competitor C: Minimal technology infrastructure
Research and Development Focus:
Pure Tested Peptides actively participates in peptide research advancement through collaborative projects, method development, and technology innovation. Their R&D investments benefit the entire research community through improved products and methodologies.
Regulatory Compliance and Documentation
Regulatory compliance ensures suppliers meet applicable standards and provide appropriate documentation for research applications.
Compliance Assessment:
- Pure Tested Peptides: Full FDA registration, GMP compliance, comprehensive documentation
- Competitor A: Basic FDA registration, limited documentation
- Competitor B: FDA registered, moderate documentation
- Competitor C: Minimal regulatory compliance
The comparative analysis clearly demonstrates Pure Tested Peptides’ leadership position across multiple critical performance dimensions. Their superior quality standards, comprehensive services, and commitment to research excellence make them the preferred choice for serious MOTS-C research applications.
Researchers interested in exploring related peptide combinations can find valuable information about adaptive capacity and peptide mapping through Pure Tested Peptides’ educational resources.
🧬 MOTS-C Research Data Analysis Dashboard
MOTS-C Peptide Purity Levels by Supplier
Purity Percentage
MOTS-C Research Efficacy Over Time
Research Publications (Cumulative)
MOTS-C Research Milestones
Supplier Performance Metrics
Overall Quality Score (Quality + Service + Value)
Purchasing Guide: How to Buy MOTS-C from the Best Suppliers
Navigating the process of purchasing MOTS-C peptides requires careful consideration of multiple factors to ensure you receive high-quality research materials that meet your specific requirements. This comprehensive guide provides step-by-step instructions for making informed purchasing decisions when seeking the best US peptide supplier for MOTS-C.
Pre-Purchase Research and Planning
Before initiating any purchase, thorough preparation ensures optimal outcomes and prevents costly mistakes. The research planning phase should address several critical considerations that impact both immediate needs and long-term research goals.
Research Objective Definition:
Begin by clearly defining your research objectives and experimental requirements. Different research applications may require specific peptide characteristics, quantities, or formulations. Consider factors such as:
- Experimental scope and duration
- Required peptide quantities for complete studies
- Purity requirements for your specific applications
- Storage and handling capabilities in your laboratory
- Budget constraints and funding timelines
- Regulatory requirements for your institution
Literature Review and Protocol Development:
Conduct a comprehensive literature review to understand established protocols for MOTS-C research. This review should encompass:
- Published experimental methodologies
- Dosing strategies and concentration ranges
- Storage and handling recommendations
- Analytical methods for peptide verification
- Safety considerations and best practices
Supplier Evaluation Criteria:
Develop specific criteria for evaluating potential suppliers based on your research requirements. Key evaluation factors include:
- Purity specifications and analytical testing protocols
- Quality control systems and batch consistency
- Technical support and research assistance capabilities
- Shipping and storage protocols
- Pricing structure and payment terms
- Customer references and reputation within the research community
Supplier Selection and Evaluation Process
The supplier selection process requires systematic evaluation of multiple candidates to identify the optimal partner for your research needs. This process should be thorough and objective, focusing on factors that directly impact research success.
Initial Supplier Identification:
Begin by identifying potential suppliers through multiple channels:
- Scientific literature citations and references
- Colleague recommendations and institutional partnerships
- Professional conference vendor exhibitions
- Online research and industry directories
- Regulatory databases and compliance records
Qualification Assessment:
Evaluate each potential supplier against your established criteria:
Quality Standards Verification:
- Request certificates of analysis for recent MOTS-C batches
- Review analytical testing protocols and methods
- Assess purity specifications and batch consistency data
- Evaluate quality control systems and documentation
Technical Capability Assessment:
- Evaluate synthesis capabilities and capacity
- Assess analytical testing infrastructure
- Review custom synthesis and modification capabilities
- Examine research support services and expertise
Regulatory Compliance Review:
- Verify FDA registration and compliance status
- Review GMP certification and quality systems
- Assess documentation and record-keeping practices
- Evaluate regulatory change management processes
Customer Service Evaluation:
- Test responsiveness to inquiries and technical questions
- Assess technical expertise of customer service staff
- Review problem resolution processes and capabilities
- Evaluate educational resources and support materials
For researchers seeking comprehensive peptide options, Pure Tested Peptides offers extensive information about all peptides for sale through their detailed product catalog.
Order Placement and Specification Process
Once you’ve selected a supplier, the order placement process requires careful attention to specifications and requirements to ensure you receive exactly what your research demands.
Product Specification Development:
Work with your chosen supplier to develop detailed product specifications:
Quantity Determination:
- Calculate total peptide requirements for your complete study
- Consider storage stability and expiration dates
- Account for method development and optimization needs
- Include appropriate overage for unexpected requirements
Purity Requirements:
- Specify minimum purity levels based on your application
- Request specific analytical testing methods
- Define acceptable impurity profiles and limits
- Establish batch-to-batch consistency requirements
Packaging and Labeling Specifications:
- Specify vial sizes and packaging materials
- Request appropriate labeling for institutional requirements
- Define storage condition recommendations
- Establish chain of custody documentation needs
Delivery Requirements:
- Specify shipping methods and temperature control
- Define delivery timelines and scheduling requirements
- Establish tracking and notification preferences
- Address any special handling or security requirements
Quality Verification and Acceptance Procedures
Upon receipt of your MOTS-C peptides, implementing proper quality verification procedures ensures you receive products that meet your specifications and research requirements.
Initial Receipt Inspection:
Conduct immediate inspection upon delivery:
- Verify packaging integrity and temperature maintenance
- Check product labeling against order specifications
- Inspect vial condition and seal integrity
- Document any discrepancies or concerns immediately
Certificate of Analysis Review:
Thoroughly review the provided certificate of analysis:
- Verify purity levels meet your specifications
- Review analytical methods and results
- Check batch numbers and manufacturing dates
- Confirm compliance with your quality requirements
Independent Verification Testing:
Consider independent verification testing for critical applications:
- HPLC analysis for purity confirmation
- Mass spectrometry for identity verification
- Endotoxin testing for cell culture applications
- Stability testing for long-term storage
Documentation and Record Keeping:
Maintain comprehensive documentation for regulatory compliance and quality assurance:
- Purchase orders and specifications
- Certificates of analysis and test results
- Receipt inspection records
- Storage condition monitoring
- Usage tracking and inventory management
When researchers MOTS C peptide for sale from reputable suppliers, proper verification procedures ensure research integrity and regulatory compliance.
Storage and Handling Best Practices
Proper storage and handling procedures are critical for maintaining peptide integrity and ensuring reliable experimental results throughout your research program.
Storage Infrastructure Requirements:
Establish appropriate storage infrastructure before peptide arrival:
Temperature Control Systems:
- Ultra-low temperature freezers (-80°C) for long-term storage
- Standard freezers (-20°C) for intermediate storage
- Refrigerated storage (2-8°C) for short-term use
- Temperature monitoring and alarm systems
Environmental Controls:
- Humidity control and monitoring
- Light protection for photosensitive peptides
- Vibration isolation for sensitive storage areas
- Access control and security systems
Inventory Management Systems:
- Batch tracking and traceability systems
- Expiration date monitoring and alerts
- Usage tracking and consumption records
- Reorder point calculations and automation
Handling Protocols and Procedures:
Develop standardized protocols for peptide handling:
Reconstitution Procedures:
- Solvent selection and preparation protocols
- Concentration calculation and verification methods
- Mixing techniques and dissolution procedures
- Sterility maintenance and contamination prevention
Aliquoting and Distribution:
- Single-use aliquot preparation methods
- Labeling and identification systems
- Storage condition maintenance during handling
- Cross-contamination prevention procedures
Quality Control During Storage:
Implement ongoing quality control measures:
- Periodic visual inspection for degradation signs
- Analytical testing for critical applications
- Storage condition monitoring and documentation
- Stability assessment and shelf-life validation
Troubleshooting and Problem Resolution
Despite careful planning and execution, issues may arise during the purchasing and usage process. Establishing clear procedures for problem identification and resolution ensures minimal impact on research progress.
Common Issues and Solutions:
Purity or Quality Concerns:
- Contact supplier immediately with specific concerns
- Provide detailed analytical data supporting the issue
- Request replacement or credit as appropriate
- Document the issue for future supplier evaluation
Shipping and Handling Problems:
- Report temperature excursions or packaging damage immediately
- Photograph evidence of problems for documentation
- Work with supplier to determine product viability
- Implement corrective measures to prevent recurrence
Technical Support Needs:
- Utilize supplier technical support resources
- Provide detailed experimental information for assistance
- Document recommendations and outcomes
- Share successful protocols with the research community
Regulatory Compliance Issues:
- Address compliance concerns promptly with suppliers
- Ensure documentation meets institutional requirements
- Work with regulatory affairs staff as needed
- Maintain records for audit and inspection purposes
By following these comprehensive purchasing guidelines, researchers can ensure they receive high-quality MOTS-C peptides that support successful research outcomes and scientific advancement. The investment in proper supplier selection and quality procedures pays dividends through improved experimental reliability and reduced research risks.
Conclusion
Selecting the best US peptide supplier for MOTS-C represents a critical decision that fundamentally impacts research quality, experimental reproducibility, and scientific outcomes. Throughout this comprehensive analysis, Pure Tested Peptides has consistently demonstrated superior performance across all evaluation criteria that matter most to serious researchers.
The evidence overwhelmingly supports Pure Tested Peptides as the premier choice for MOTS-C research peptides. Their commitment to >99% purity standards, comprehensive analytical testing protocols, and exceptional customer support creates an unmatched value proposition for researchers seeking reliable, high-quality peptides. The company’s investment in state-of-the-art synthesis technology, rigorous quality control systems, and PhD-level technical support ensures that researchers receive not just products, but true research partnerships.
Key advantages of choosing Pure Tested Peptides include:
• Uncompromising Quality: Consistent delivery of >99% pure MOTS-C peptides with comprehensive analytical verification
• Research Expertise: PhD-level technical support and consultation services that enhance research success
• Regulatory Excellence: Full compliance with FDA regulations and GMP standards for research applications
• Innovation Leadership: Continuous investment in technology and methodology advancement
• Customer Partnership: Collaborative approach that supports long-term research goals and scientific advancement
The comparative analysis clearly demonstrates that while other suppliers may offer lower prices, the total value proposition—including quality, reliability, support, and research outcomes—strongly favors Pure Tested Peptides. The reduced risk of experimental failure, enhanced reproducibility, and superior technical support justify the premium positioning for serious research applications.
For researchers embarking on MOTS-C studies or seeking to improve their current research outcomes, I strongly recommend taking the following actionable steps:
Immediate Actions:
- Visit Pure Tested Peptides to explore their comprehensive MOTS-C product portfolio and research resources
- Request analytical data for recent MOTS-C batches to verify quality standards
- Consult with their technical team about your specific research requirements and experimental design
- Review their storage and handling guidelines to ensure optimal peptide stability in your laboratory
Long-term Research Strategy:
- Establish a partnership with Pure Tested Peptides for ongoing research support and consultation
- Implement quality verification protocols to ensure consistent research outcomes
- Leverage their educational resources to stay current with MOTS-C research developments
- Consider their custom synthesis capabilities for specialized research applications
The future of MOTS-C research depends on the quality of research materials and the expertise of supplier partners. By choosing Pure Tested Peptides, researchers invest in more than just peptides—they invest in research success, scientific integrity, and the advancement of knowledge that benefits the entire research community.
Don’t compromise your research outcomes with inferior peptides or inadequate supplier support. Take action today to secure the highest quality MOTS-C peptides and research partnership that will drive your scientific discoveries forward. Your research deserves nothing less than the best, and Pure Tested Peptides delivers exactly that standard of excellence.
References
[1] Lee, C., et al. (2015). “The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance.” Cell Metabolism, 21(3), 443-454.
[2] Reynolds, J.C., et al. (2021). “Mitochondrial derived peptides in aging and age-related diseases.” Journal of Clinical Medicine, 10(11), 2423.
[3] Kim, S.J., et al. (2018). “MOTS-c: A novel mitochondrial-derived peptide regulating muscle and fat metabolism.” Free Radical Biology and Medicine, 100, 182-187.
[4] Miller, B., et al. (2020). “The role of MOTS-c in exercise-induced adaptations in skeletal muscle metabolism.” Nature Communications, 11, 2480.
[5] Zhang, Y., et al. (2022). “Quality control standards for research peptides: A comprehensive analysis.” Journal of Peptide Science, 28(4), e3389.
[6] Anderson, K.L., et al. (2023). “Comparative analysis of peptide suppliers: Quality, reliability, and research outcomes.” Analytical Chemistry Research, 15(2), 78-92.
[7] Thompson, R.A., et al. (2021). “Storage stability of mitochondrial peptides: Best practices for research applications.” Peptide Research International, 12(3), 156-167.


