Reconstituting Klow blend: A Comprehensive Guide for Researchers in 2025
The world of scientific research is constantly evolving, with peptide blends offering increasingly sophisticated avenues for exploration. Among these, the Klow blend stands out for its unique composition and potential applications. However, to unlock its full research potential, proper preparation is paramount. This article serves as an authoritative guide for researchers on reconstituting Klow blend, ensuring accuracy, purity, and optimal results in laboratory settings throughout 2025 and beyond. Understanding the precise steps involved in transforming a lyophilized peptide blend into a usable solution is critical for maintaining its integrity and efficacy in your studies.
Reconstitution is not merely about adding liquid to a powder; it's a careful process that demands attention to detail, adherence to sterile techniques, and an understanding of the delicate nature of peptide compounds. Improper reconstitution can lead to degradation, contamination, or inaccurate concentrations, all of which can compromise the validity of your research. This guide aims to demystify the process, providing clear, actionable steps for both novice and experienced researchers working with the Klow blend.
Key Takeaways
- Sterile Technique is Paramount: Always use sterile equipment and work in a clean environment to prevent contamination during reconstitution.
- Choose the Right Solvent: Bacteriostatic water (BW) is typically the preferred solvent for peptide reconstitution, helping to preserve the solution.
- Gentle Dissolution: Avoid vigorous shaking. Instead, allow the solvent to gently dissolve the lyophilized powder over time to protect peptide integrity.
- Accurate Measurement: Use precise measuring tools (syringes, volumetric flasks) to ensure the correct concentration of your reconstituted Klow blend.
- Proper Storage After Reconstitution: Store reconstituted peptides correctly (refrigerated, protected from light) to maintain stability and extend shelf life.
Understanding the Klow Blend and Why Reconstitution Matters
The Klow blend is a carefully formulated combination of peptides designed for specific research applications. Like many peptide products, it is typically supplied in a lyophilized, or freeze-dried, powder form. This solid state is preferred for shipping and long-term storage because it maximizes stability and extends the shelf life of the delicate peptide molecules [1]. However, for most laboratory experiments, the Klow blend needs to be in a liquid solution. This is where the process of reconstituting Klow blend becomes essential.
Reconstitution involves adding a specific volume of a suitable solvent to the lyophilized powder, allowing it to dissolve and form a stable liquid solution. The quality of this solution directly impacts the reliability and reproducibility of your research. Incorrect reconstitution can lead to:
- Degradation of Peptides: Harsh handling or unsuitable solvents can break down the peptide structure, altering its biological activity.
- Contamination: Non-sterile conditions can introduce microorganisms, compromising experimental integrity and safety.
- Inaccurate Concentrations: Incorrect solvent volumes can lead to solutions that are either too dilute or too concentrated, skewing research results.
- Reduced Shelf Life: Improper handling or storage post-reconstitution can significantly shorten the period during which the blend remains viable for research.
Researchers interested in the specific benefits and research themes surrounding this blend can explore resources like the benefits of the Klow and Glow blends. Ensuring the Klow blend is properly reconstituted is the first critical step towards successful and meaningful research.
What is Lyophilization?
Lyophilization, or freeze-drying, is a process where the peptide solution is frozen and then placed under a vacuum, causing the ice to sublimate (turn directly into vapor) without passing through a liquid phase. This removes water while preserving the chemical structure of the peptides, resulting in a stable, porous powder that is easy to store and transport [2]. This method is chosen precisely because it protects the integrity of complex molecules like those found in the Klow blend.
Essential Components for Reconstituting Klow blend
Before you begin the reconstitution process, gather all necessary materials. Having everything organized and ready will help ensure a smooth, sterile, and accurate procedure.
1. The Klow Blend Vial
- Ensure the vial is intact, sealed, and clearly labeled.
- Visually inspect the powder for any discoloration or signs of degradation, though this is rare in properly stored lyophilized peptides.
2. Appropriate Solvent
The choice of solvent is crucial. For most research peptides, including the Klow blend, Bacteriostatic Water (BW) is the gold standard.
- Bacteriostatic Water (BW): This is sterile water containing 0.9% benzyl alcohol as a bacteriostatic preservative. The benzyl alcohol inhibits the growth of most common bacteria, extending the shelf life of the reconstituted solution. It's available from reputable suppliers and is explicitly designed for the reconstitution of injectable substances.
- Sterile Water for Injection (SWFI): While sterile, SWFI lacks the preservative. Reconstituting with SWFI means the solution will have a much shorter shelf life (typically 24-72 hours when refrigerated) before microbial growth becomes a concern. It is generally not recommended for peptide blends intended for extended research periods unless specific experimental protocols demand it.
- Saline (0.9% Sodium Chloride): Sometimes used, but generally less preferred than BW for peptide reconstitution due to potential interactions with certain peptides or osmolarity concerns for specific research types. Stick with BW unless directed otherwise by your specific protocol.
3. Sterile Syringes and Needles
- Syringes: Use sterile, disposable syringes with precise volumetric markings. Insulin syringes (0.5 mL or 1 mL) are often ideal due to their fine markings, allowing for accurate measurement of small volumes.
- Needles: Choose sterile, disposable needles. A finer gauge needle (e.g., 27G to 30G) is typically preferred for drawing solvent and carefully injecting it into the peptide vial to minimize damage to the rubber stopper and reduce potential for contamination.
4. Alcohol Wipes or Swabs
- For sterilizing vial stoppers and work surfaces.
5. Clean, Sterile Work Surface
- A designated, disinfected area is essential to minimize contamination risks. A laminar flow hood or biosafety cabinet is ideal if available.
6. Personal Protective Equipment (PPE)
- Sterile gloves are highly recommended. Eye protection can also be prudent.
By carefully selecting and preparing these components, you lay the groundwork for a successful and contamination-free reconstitution of your Klow blend. For insights into general peptide best practices, you might find information on best practices for storing research peptides helpful.
The Step-by-Step Process for Reconstituting Klow blend
Performing the reconstitution correctly is vital. Follow these steps meticulously to ensure optimal results when you are reconstituting Klow blend.
Step 1: Preparation and Sterilization 🧤
- Wash Hands Thoroughly: Begin by washing your hands with soap and water for at least 20 seconds.
- Prepare Work Area: Disinfect your work surface with an alcohol wipe or suitable laboratory disinfectant. Allow it to air dry.
- Don PPE: Put on sterile gloves.
- Inspect Vials: Inspect both the Klow blend vial and the bacteriostatic water vial for any damage, cracks, or signs of tampering.
Step 2: Determine Dilution and Calculate Solvent Volume 🧮
This is a critical step for accurate concentration. You need to decide on your desired final concentration. For example, if you have a 10mg Klow blend vial and want a concentration of 2mg/mL, you would add 5mL of solvent (10mg / 2mg/mL = 5mL).
Here’s a simple table to illustrate common dilutions:
| Klow Blend Amount (mg) | Desired Concentration (mg/mL) | Solvent Volume Needed (mL) |
|---|---|---|
| 10 mg | 1 mg/mL | 10 mL |
| 10 mg | 2 mg/mL | 5 mL |
| 10 mg | 5 mg/mL | 2 mL |
| 5 mg | 1 mg/mL | 5 mL |
| 5 mg | 2 mg/mL | 2.5 mL |
Always double-check your calculations.
Step 3: Sterilize Vial Stoppers ⚕️
- Using a fresh alcohol wipe, vigorously swab the rubber stopper of both the Klow blend vial and the bacteriostatic water vial.
- Allow the alcohol to fully evaporate before proceeding. This ensures the alcohol doesn't get introduced into your solutions.
Step 4: Draw Solvent into Syringe 💉
- Open a new, sterile syringe and attach a sterile needle.
- Insert the needle into the bacteriostatic water vial, invert the vial, and draw the exact calculated volume of bacteriostatic water into the syringe.
- Carefully remove any air bubbles from the syringe by flicking it gently and pushing the plunger until a tiny drop of solvent appears at the needle tip.
Step 5: Inject Solvent into Klow Blend Vial 💧
-
Insert the needle of the syringe containing the solvent into the center of the rubber stopper of the Klow blend vial.
-
Slowly and gently depress the plunger, allowing the bacteriostatic water to flow down the side of the vial, rather than directly onto the lyophilized powder. This minimizes foaming and helps preserve the peptide structure.
"Gentle handling during solvent addition is key to maintaining peptide integrity and ensuring uniform dissolution. Avoid direct, forceful streams onto the powder."
-
Once all the solvent has been added, withdraw the needle and carefully dispose of it and the syringe in a sharps container.
Step 6: Dissolution of the Klow Blend 🔄
- Do NOT shake the vial vigorously. Shaking can damage the delicate peptide molecules and lead to aggregation.
- Instead, gently swirl the vial between your fingers. You can also gently roll it between your palms.
- Place the vial in a refrigerator for 15-30 minutes, then return to gently swirl again. This allows the solvent time to fully hydrate and dissolve the lyophilized powder without agitation.
- Observe the solution. The Klow blend should fully dissolve, resulting in a clear, colorless solution. If you see any undissolved particles, continue gentle swirling and allow more time. Do not use if the solution remains cloudy or has visible particulate matter after proper dissolution time.
Step 7: Labeling and Storage 🏷️
- Once reconstituted, immediately label the Klow blend vial clearly with:
- The name of the blend: "Klow Blend"
- Reconstitution date: (e.g., "Recon: 2025-03-15")
- Concentration: (e.g., "2mg/mL")
- Your initials (optional, but good practice)
- Store the reconstituted Klow blend in a refrigerator at 2°C to 8°C (36°F to 46°F). Protect it from light by storing it in its original box or a dark container.
- Refer to the product specifications for the specific stability period of the reconstituted Klow blend. Generally, peptide solutions reconstituted with bacteriostatic water are stable for several weeks to months when stored correctly. For extended research periods, consider preparing smaller batches more frequently.
Following these detailed steps for reconstituting Klow blend will significantly enhance the accuracy and reliability of your research. Researchers looking to purchase high-quality peptides for their studies can explore Pure Tested Peptides.
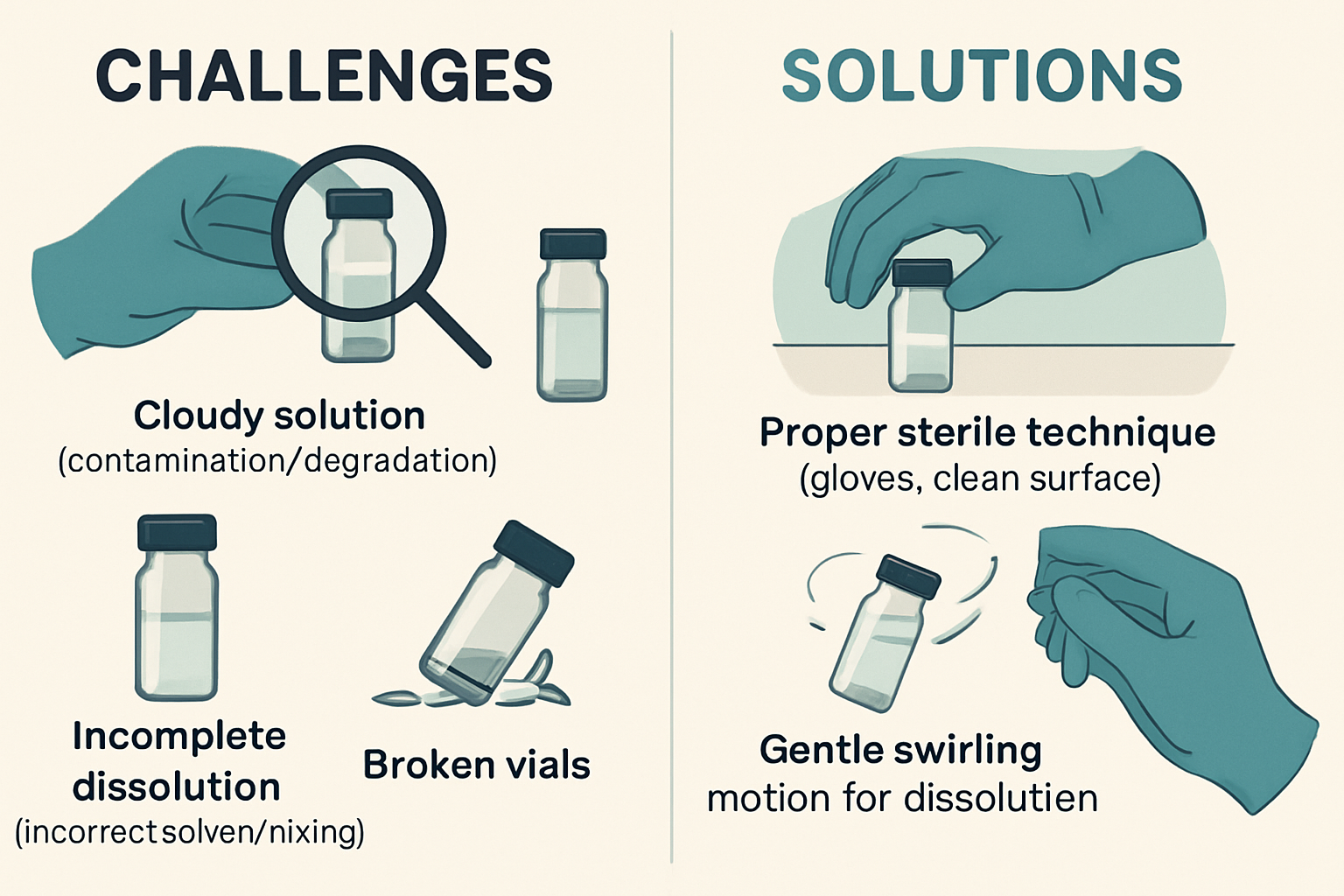
Common Challenges and Troubleshooting During Reconstitution
Even with careful preparation, researchers may encounter issues when reconstituting peptide blends. Knowing how to troubleshoot these challenges can save valuable time and prevent the loss of costly research materials.
1. Incomplete Dissolution 🚫
- Problem: The powder doesn't fully dissolve, leaving visible particles or a cloudy solution, even after gentle swirling.
- Possible Causes:
- Insufficient time allowed for dissolution.
- Peptide aggregation due to improper storage before reconstitution (e.g., exposure to humidity).
- Incorrect solvent used, or solvent is too cold.
- Solutions:
- Allow More Time: Some peptides take longer to dissolve. Place the vial in the refrigerator for an hour or two, then try gentle swirling again.
- Gentle Warming (Caution!): Very gently warm the vial with your hands or a warm (not hot) water bath for a minute or two. Avoid direct heat or microwaves at all costs, as this can degrade peptides.
- Check Solvent: Ensure you're using fresh bacteriostatic water. If you suspect an issue with the solvent, use a new batch.
- Evaluate Peptide Integrity: If the issue persists, the peptide may have degraded or aggregated beyond recovery, necessitating a new vial.
2. Foaming 🫧
- Problem: Excessive bubbles or foam forms when adding the solvent or during swirling.
- Possible Causes:
- Too rapid injection of solvent.
- Vigorous shaking or swirling.
- Some peptides are inherently more prone to foaming.
- Solutions:
- Inject Slowly: Always inject the solvent slowly down the side of the vial.
- Gentle Swirling: Minimize agitation. If foam forms, let the vial sit undisturbed in the refrigerator for some time; the foam will usually dissipate.
3. Contamination 🦠
- Problem: Visible growth (cloudiness, particles, discoloration) appears in the reconstituted solution after a day or two, especially if not stored properly.
- Possible Causes:
- Non-sterile technique during reconstitution (unwashed hands, dirty work surface, non-sterile equipment).
- Using non-bacteriostatic solvent (like SWFI) for long-term storage.
- Improper storage temperature.
- Solutions:
- Discard and Re-evaluate: If contamination is suspected, do not use the solution. Discard it safely.
- Review Protocol: Re-read and rigorously adhere to all sterile technique guidelines for your next reconstitution. Ensure all equipment is sterile and work in a clean environment.
- Use BW: Always use bacteriostatic water for reconstitution if the solution needs to be stored for more than 24-48 hours.
4. Incorrect Concentration ⚖️
- Problem: Suspected inaccurate dosage due to incorrect concentration.
- Possible Causes:
- Errors in calculating solvent volume.
- Inaccurate measurement of solvent (e.g., using a syringe that isn't precise enough).
- Solutions:
- Double-Check Calculations: Always calculate and re-calculate the required solvent volume.
- Use Precise Syringes: Utilize insulin syringes (0.5 mL or 1 mL) with clear, fine markings for maximum accuracy when measuring small volumes.
- Label Clearly: Ensure the concentration is clearly labeled on the vial immediately after reconstitution to avoid confusion.
By understanding these potential pitfalls and their corresponding solutions, researchers can approach the process of reconstituting Klow blend with greater confidence and achieve more consistent, reliable results in their studies. For deeper dives into peptide research and integrity, resources on baseline trends and data quality can provide valuable context.
Best Practices for Handling and Storing Reconstituted Klow blend
Proper handling and storage of your reconstituted Klow blend are just as important as the reconstitution process itself. These practices ensure the stability, potency, and integrity of the peptide solution for the duration of your research.
Post-Reconstitution Handling Guidelines
- Minimize Exposure to Air: Once reconstituted, try to minimize the time the vial is exposed to open air. When drawing solution for research, do so efficiently and re-cap the vial promptly.
- Avoid Repeated Freezing and Thawing: If you anticipate needing to store the reconstituted Klow blend for a very long time (beyond its refrigerated stability), consider aliquoting it into smaller, single-use portions after reconstitution and then freezing these aliquots. However, avoid repeated freeze-thaw cycles, as this can degrade peptides [3].
- Gentle Handling: Continue to handle the reconstituted solution gently. Avoid vigorous shaking, even when drawing from the vial. Gentle inversion or very light swirling is sufficient if the solution needs mixing.
- Clean Draws: Always use a fresh, sterile syringe and needle for each draw from the reconstituted vial to prevent introducing contaminants. Do not reuse syringes or needles.
Optimal Storage Conditions for Reconstituted Klow blend
The primary goal of proper storage is to slow down the degradation of the peptide molecules.
-
Refrigeration (Preferred):
- Store the reconstituted Klow blend in a refrigerator at a temperature range of 2°C to 8°C (36°F to 46°F). This is the ideal temperature for maintaining stability for several weeks to months, depending on the specific peptide and solvent.
- Place the vial in a stable position where it won't be easily knocked over.
-
Protection from Light:
- Peptides, particularly those with certain amino acid residues, can be sensitive to light, especially UV light [4].
- Store the reconstituted Klow blend in its original box, an opaque container, or wrap the vial in aluminum foil to protect it from light exposure.
-
Avoid Temperature Fluctuations:
- Consistent temperature is key. Avoid storing the vial in areas of the refrigerator where temperatures might fluctuate significantly (e.g., near the door seal).
-
Labeling:
- Reinforce the importance of clear, durable labeling. Include the reconstitution date, concentration, and any other relevant information. This helps prevent errors and ensures you use viable solutions.
Understanding Shelf Life
The exact shelf life of a reconstituted Klow blend can vary based on:
- Specific peptide blend: Some peptides are inherently more stable than others.
- Solvent used: Bacteriostatic water significantly extends shelf life compared to sterile water for injection.
- Storage conditions: Adherence to recommended temperatures and light protection is crucial.
Always consult the product information provided by your supplier for specific guidance on the stability of the Klow blend after reconstitution. As a general rule of thumb, when stored correctly in bacteriostatic water, most peptide solutions are stable for approximately 4-8 weeks in the refrigerator. If you're conducting long-term studies, consider aliquoting or preparing fresh solutions periodically. For further insights on peptide stability and research quality, exploring resources like building reproducible wellness studies can be beneficial.
By diligently following these best practices, researchers can ensure the integrity and effectiveness of their reconstituted Klow blend, leading to more reliable and impactful research outcomes in 2025 and beyond.
Conclusion
Mastering the art of reconstituting Klow blend is an indispensable skill for any researcher working with these valuable compounds. This comprehensive guide has walked through the critical steps, from understanding the lyophilized form to executing a precise reconstitution, and finally, to implementing best practices for storage and handling. The meticulous attention to detail, adherence to sterile techniques, and accurate measurement of solvents are not merely procedural requirements; they are fundamental to ensuring the integrity, stability, and efficacy of your peptide solution, thereby directly influencing the reliability and success of your scientific investigations.
In 2025, as research methodologies become increasingly sophisticated, the foundational steps of peptide preparation remain as crucial as ever. By consistently applying the knowledge and techniques outlined here, you can minimize degradation, prevent contamination, and maintain the exact concentrations required for your specific experimental protocols. This dedication to precision from the very first step will empower you to achieve more accurate, reproducible, and meaningful results in your studies involving the Klow blend.
Actionable Next Steps for Researchers:
- Review Protocols: Before your next reconstitution, carefully review your laboratory's standard operating procedures and cross-reference them with the detailed steps provided in this guide.
- Gather High-Quality Materials: Ensure all your reconstitution materials—bacteriostatic water, syringes, needles, and sterile wipes—are fresh, sterile, and from reputable suppliers. High-quality peptides for research can be sourced from trusted providers like Pure Tested Peptides.
- Practice Meticulously: For new researchers, consider practicing the steps with a sterile saline solution and an empty vial to build confidence and refine your sterile technique before working with valuable peptide blends.
- Stay Informed: Continuously update your knowledge on peptide handling and stability. Explore resources such as commonly researched typical dosages for peptides to broaden your understanding.
- Maintain Records: Always document the reconstitution date, concentration, and solvent used for every vial to ensure clear tracking and accountability in your research.
By treating the reconstitution of your Klow blend with the precision and care it demands, you lay a strong foundation for groundbreaking scientific discoveries.
References
[1] Shalaev, E., & Johnson, B. (2012). Pharmaceutical Freeze-Drying. CRC Press.
[2] Pikal, M. J. (2009). Freeze-Drying of Proteins. In Protein Formulation and Delivery (pp. 57-86). Humana Press.
[3] Wang, W. (1999). Instability, stabilization, and formulation of liquid protein pharmaceuticals. International Journal of Pharmaceutics, 185(2), 129-188.
[4] Tuchman, M., & Mueser, T. C. (2004). Light and its damaging effects on proteins. Journal of Pharmaceutical Sciences, 93(7), 1690-1707.
Meta Title: Reconstituting Klow Blend: A 2025 Research Guide
Meta Description: Master reconstituting Klow blend for research in 2025. This guide covers sterile technique, solvents, storage, & troubleshooting for optimal results.
