Understanding tesa Side Effects: A Comprehensive Guide for 2025

When researchers first developed tesa as a synthetic growth hormone-releasing hormone (GHRH) analog, they uncovered a powerful peptide that would revolutionize approaches to growth hormone deficiency and lipodystrophy research. However, like all bioactive compounds, understanding tesa side effects is crucial for anyone considering its use in research applications or clinical settings.
Key Takeaways
• Common side effects include injection site reactions, joint pain, and mild gastrointestinal symptoms that typically resolve with continued use
• Serious adverse effects are rare but can include glucose intolerance and potential tumor growth stimulation in predisposed individuals
• Individual responses vary significantly based on dosage, duration of use, and personal health factors
• Proper monitoring and medical supervision are essential for safe tesa administration
• Drug interactions and contraindications must be carefully evaluated before starting tesa therapy
What Is tesa and How Does It Work?
tesa is a synthetic analog of growth hormone-releasing hormone (GHRH) that stimulates the anterior pituitary gland to produce and release growth hormone. Originally developed for treating HIV-associated lipodystrophy, this 44-amino acid peptide has gained attention in research circles for its potential applications in various metabolic conditions.
The mechanism of action involves binding to GHRH receptors in the pituitary gland, triggering a cascade that ultimately increases growth hormone production. This process can lead to improved body composition, reduced visceral fat, and enhanced metabolic function. However, these benefits come with a spectrum of potential side effects that researchers and clinicians must carefully consider.
For those interested in exploring tesa research applications, understanding the complete safety profile is essential for responsible use.
Common tesa Side Effects: What to Expect
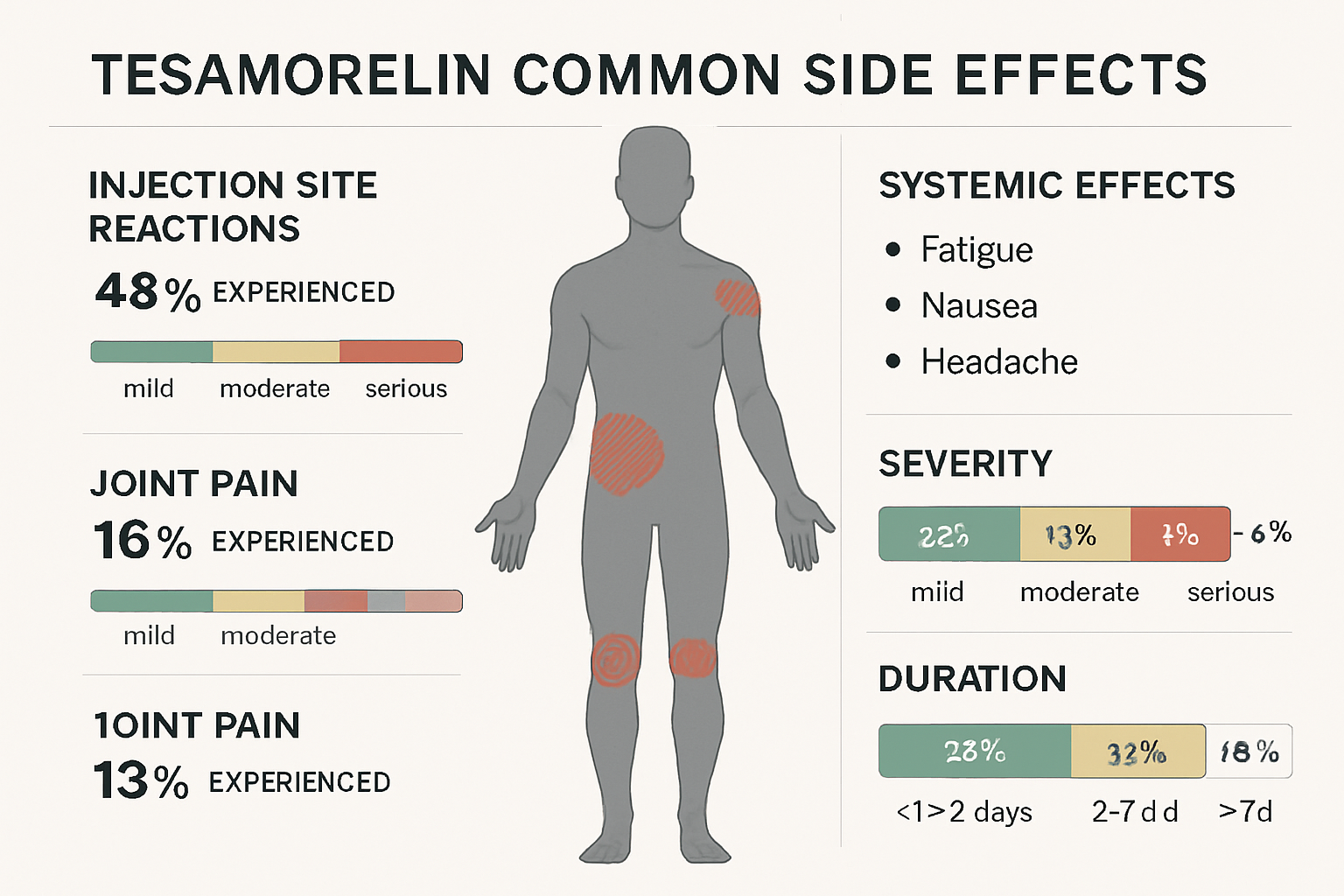
Injection Site Reactions
The most frequently reported tesa side effects occur at the injection site. These local reactions affect approximately 30-40% of users and typically include:
- Redness and swelling at the injection point
- Pain or tenderness lasting 24-48 hours
- Bruising that may persist for several days
- Nodule formation in some cases with repeated injections
Most injection site reactions are mild to moderate and tend to decrease in frequency and severity as the body adapts to regular administration. Proper injection technique and site rotation can significantly reduce these occurrences.
Joint and Muscle Symptoms
Research indicates that joint-related side effects occur in roughly 20-25% of tesa users. These symptoms commonly manifest as:
- Arthralgia (joint pain) affecting multiple joints
- Muscle stiffness particularly in the morning
- Carpal tunnel syndrome symptoms in some individuals
- General muscle aches that may mimic flu-like symptoms
These effects are thought to result from increased growth hormone levels affecting connective tissue and fluid retention patterns. The symptoms often improve with dose adjustments or temporary treatment interruption.
Gastrointestinal Effects
Digestive system side effects represent another category of common tesa reactions, affecting approximately 15-20% of users:
- Nausea especially during the initial weeks of treatment
- Abdominal discomfort or cramping
- Changes in appetite which may increase or decrease
- Mild diarrhea in some individuals
These gastrointestinal symptoms typically resolve as the body adjusts to the peptide, usually within 2-4 weeks of consistent use.
For researchers studying peptide interactions and combinations, understanding how tesa affects different body systems is crucial for comprehensive research design.
Serious tesa Side Effects and Safety Concerns
Glucose Metabolism Disruption
One of the most significant concerns with tesa use involves its potential impact on glucose homeostasis. Studies have documented several glucose-related complications:
Insulin Resistance Development
- Increased fasting glucose levels in 10-15% of users
- Reduced insulin sensitivity over time
- Potential progression to type 2 diabetes in predisposed individuals
Diabetic Complications
- Worsening glycemic control in existing diabetics
- Increased risk of diabetic ketoacidosis
- Need for medication adjustments in diabetic patients
Regular glucose monitoring is essential for anyone using tesa, particularly those with pre-existing metabolic conditions or family history of diabetes.
Tumor Growth Stimulation
Perhaps the most serious potential tesa side effect involves the stimulation of tumor growth. Growth hormone can promote cell proliferation, raising concerns about:
Existing Malignancies
- Acceleration of known tumor growth
- Increased risk of cancer recurrence
- Particular concern with hormone-sensitive cancers
Benign Growths
- Enlargement of existing benign tumors
- Potential for benign growths to become problematic
- Increased monitoring requirements
This risk necessitates thorough cancer screening before initiating tesa therapy and ongoing surveillance throughout treatment.
Cardiovascular Considerations
While less common, cardiovascular side effects have been reported and include:
- Fluid retention leading to peripheral edema
- Blood pressure changes in some individuals
- Potential cardiac stress in those with existing heart conditions
Endocrine System Disruption
tesa can affect various hormonal pathways beyond growth hormone:
- Thyroid function alterations requiring monitoring
- Cortisol level changes affecting stress response
- Sex hormone fluctuations impacting reproductive health
When conducting comprehensive peptide research, these endocrine interactions must be carefully documented and monitored.
Factors Influencing tesa Side Effects
Dosage and Administration
The relationship between dose and side effects follows a predictable pattern:
Low Doses (1-2mg daily)
- Minimal injection site reactions
- Rare systemic effects
- Lower efficacy but improved tolerance
Standard Doses (2-3mg daily)
- Moderate side effect profile
- Balanced risk-benefit ratio
- Most commonly prescribed range
High Doses (>3mg daily)
- Increased frequency of all side effects
- Higher risk of serious complications
- Generally not recommended for routine use
Duration of Treatment
The timeline of tesa side effects varies significantly:
Initial Phase (Weeks 1-4)
- Highest incidence of injection site reactions
- Peak gastrointestinal symptoms
- Adaptation period for most users
Maintenance Phase (Months 2-6)
- Stabilization of most side effects
- Emergence of metabolic changes
- Optimal monitoring period
Long-term Use (>6 months)
- Potential for cumulative effects
- Increased cancer surveillance needs
- Regular comprehensive health assessments
Individual Risk Factors
Several personal factors influence tesa side effect profiles:
Age-Related Considerations
- Older adults show increased sensitivity
- Recovery from side effects may be slower
- Higher baseline risk for complications
Pre-existing Health Conditions
- Diabetes significantly increases metabolic risks
- Cancer history contraindicates use
- Cardiovascular disease requires careful monitoring
Genetic Factors
- Growth hormone receptor variations affect response
- Metabolic enzyme differences influence clearance
- Family history impacts risk assessment
For those exploring advanced peptide research protocols, understanding these individual variables is essential for safe and effective study design.
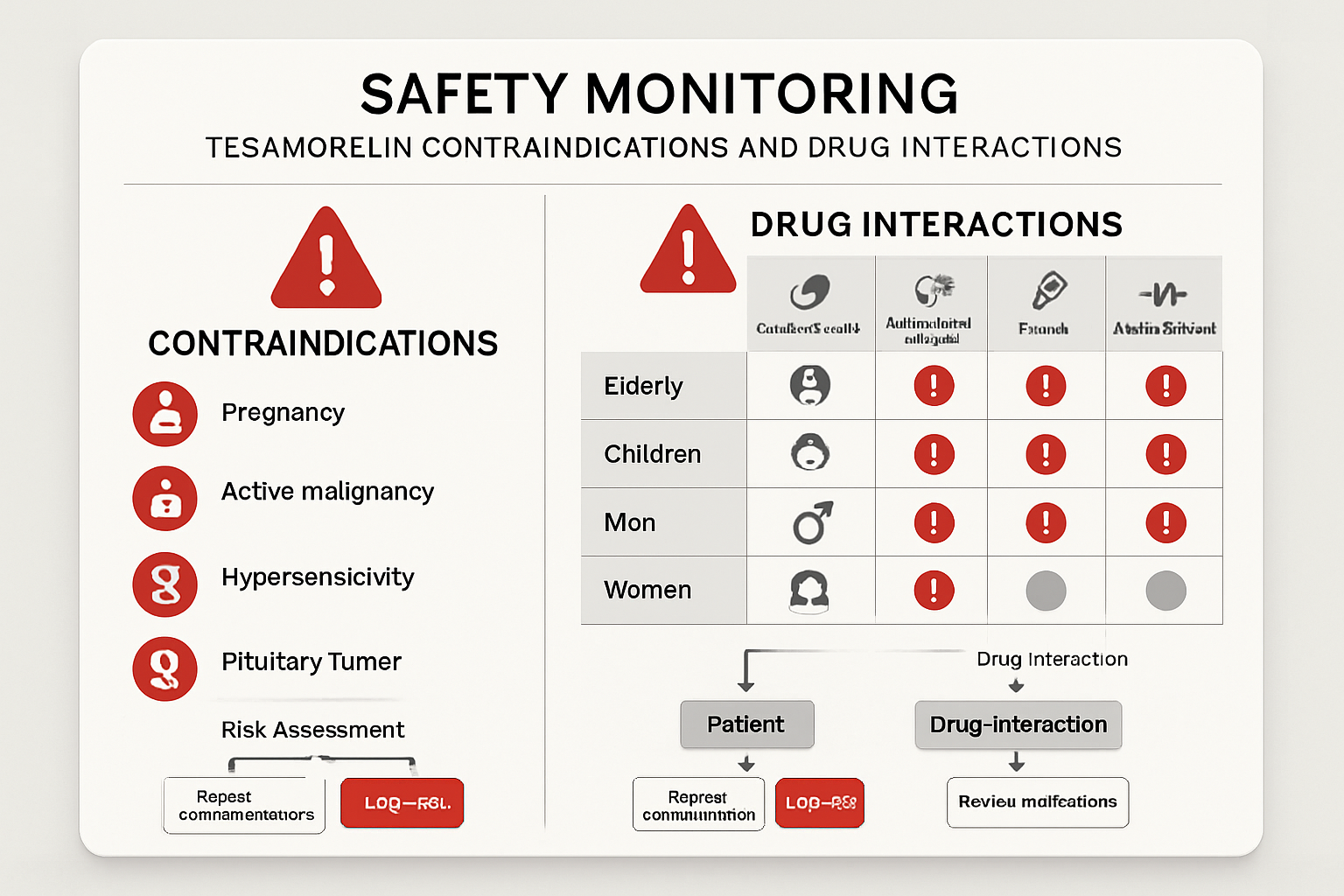
Drug Interactions and Contraindications
Medication Interactions
tesa can interact with various medications, potentially altering their effectiveness or increasing side effect risks:
Diabetes Medications
- Insulin requirements may increase
- Oral hypoglycemic agents may need adjustment
- Continuous glucose monitoring recommended
Corticosteroids
- May blunt tesa's growth hormone response
- Increased risk of glucose intolerance
- Potential for enhanced side effects
Thyroid Hormones
- tesa may affect thyroid hormone metabolism
- Dose adjustments may be necessary
- Regular thyroid function monitoring required
Absolute Contraindications
Certain conditions make tesa use inadvisable:
- Active malignancy of any type
- Acute critical illness following surgery or trauma
- Known hypersensitivity to tesa or its components
- Pregnancy and breastfeeding due to unknown effects
Relative Contraindications
These conditions require careful risk-benefit analysis:
- Diabetes mellitus with poor glycemic control
- History of malignancy within the past 5 years
- Severe cardiovascular disease
- Active inflammatory conditions
Monitoring and Management Strategies
Essential Laboratory Monitoring
Regular monitoring helps detect tesa side effects early:
Baseline Assessments
- Complete blood count and comprehensive metabolic panel
- Fasting glucose and HbA1c
- IGF-1 levels and growth hormone stimulation test
- Comprehensive cancer screening
Ongoing Monitoring Schedule
| Timepoint | Tests Required | Frequency |
|---|---|---|
| Month 1 | Glucose, liver function | Weekly |
| Month 2-3 | Full metabolic panel | Bi-weekly |
| Month 4-6 | IGF-1, glucose tolerance | Monthly |
| >6 months | Comprehensive assessment | Quarterly |
Side Effect Management Protocols
Injection Site Reactions
- Rotate injection sites systematically
- Apply ice before and after injection
- Use proper sterile technique
- Consider topical treatments for persistent reactions
Metabolic Side Effects
- Implement dietary modifications
- Increase physical activity as tolerated
- Consider dose reduction if severe
- Add glucose-lowering medications if necessary
Joint and Muscle Symptoms
- Gentle stretching and mobility exercises
- Anti-inflammatory medications as needed
- Physical therapy consultation for persistent symptoms
- Temporary dose reduction in severe cases
Researchers working with comprehensive peptide libraries should establish clear protocols for monitoring and managing side effects in their study populations.
Special Populations and Considerations
Elderly Patients
Older adults require special consideration when using tesa:
- Increased sensitivity to growth hormone effects
- Higher baseline risk for glucose intolerance
- Slower recovery from side effects
- More frequent monitoring requirements
Patients with Comorbidities
HIV-Associated Lipodystrophy
- Primary indication for tesa use
- Potential interactions with antiretroviral medications
- Enhanced monitoring for metabolic complications
- Consideration of immune system effects
Metabolic Syndrome
- Careful glucose monitoring essential
- Potential for both beneficial and harmful effects
- Individualized risk-benefit assessment
- Close collaboration with endocrinologists
Research Applications
In research settings, additional considerations apply:
- Informed consent must thoroughly cover side effect risks
- Institutional review board approval required for human studies
- Adverse event reporting protocols must be established
- Data safety monitoring boards may be necessary
When designing research protocols with peptides, comprehensive safety planning is essential for ethical and effective studies.
Long-term Safety and Surveillance
Extended Use Considerations
Long-term tesa use raises additional safety concerns:
Cancer Surveillance
- Annual comprehensive cancer screening
- Increased vigilance for new growths
- Regular imaging studies as appropriate
- Immediate discontinuation if malignancy develops
Metabolic Health
- Quarterly glucose tolerance testing
- Annual cardiovascular risk assessment
- Bone density monitoring
- Liver function surveillance
Quality of Life Monitoring
- Regular assessment of treatment benefits
- Evaluation of side effect burden
- Patient-reported outcome measures
- Periodic treatment holidays to assess necessity
Discontinuation Considerations
Stopping tesa requires careful planning:
Gradual Tapering
- Sudden discontinuation may cause rebound effects
- Gradual dose reduction over 2-4 weeks
- Monitoring for withdrawal symptoms
- Assessment of underlying condition status
Post-Treatment Monitoring
- Continued glucose monitoring for several months
- Assessment of lipodystrophy progression
- Evaluation of sustained benefits
- Planning for alternative treatments if needed
Future Research and Safety Updates
Emerging Safety Data
Ongoing research continues to refine our understanding of tesa side effects:
- Long-term cardiovascular outcomes studies in progress
- Cancer risk assessment with extended follow-up
- Pediatric safety data being collected
- Combination therapy safety profiles under investigation
Regulatory Updates
Regulatory agencies continue to monitor tesa safety:
- FDA post-market surveillance ongoing
- International safety database contributions
- Periodic safety updates from manufacturers
- Risk evaluation and mitigation strategies refinement
For researchers staying current with peptide safety developments, regular review of emerging safety data is essential.
Conclusion
Understanding tesa side effects is crucial for anyone considering its use in research or clinical applications. While this synthetic GHRH analog offers significant potential benefits for certain conditions, particularly HIV-associated lipodystrophy, its use must be balanced against a spectrum of potential adverse effects ranging from mild injection site reactions to serious metabolic and oncological concerns.
The most common tesa side effects—injection site reactions, joint symptoms, and gastrointestinal disturbances—are generally manageable with proper technique and supportive care. However, the potential for serious complications, including glucose intolerance and tumor growth stimulation, necessitates careful patient selection, comprehensive baseline assessment, and ongoing monitoring throughout treatment.
Key action steps for safe tesa use include:
- Thorough medical evaluation before initiating treatment, including cancer screening and metabolic assessment
- Establishment of regular monitoring protocols with appropriate laboratory tests and clinical evaluations
- Implementation of proper injection techniques and site rotation to minimize local reactions
- Development of side effect management strategies tailored to individual patient needs
- Maintenance of open communication between patients and healthcare providers regarding any concerning symptoms
As research continues to expand our understanding of tesa's long-term safety profile, staying informed about emerging data and regulatory updates remains essential. For researchers and clinicians working with this peptide, prioritizing safety through comprehensive monitoring and evidence-based protocols will ensure the best possible outcomes while minimizing risks.
The future of tesa research looks promising, with ongoing studies exploring new applications and refining safety protocols. By maintaining a thorough understanding of its side effect profile and implementing appropriate safeguards, researchers can continue to unlock the therapeutic potential of this important peptide while protecting the safety and well-being of study participants and patients.
References
[1] Falutz, J., et al. (2010). Effects of tesa on body composition in HIV-infected patients with lipodystrophy. AIDS, 24(14), 2169-2177.
[2] Stanley, T. L., et al. (2014). Effects of tesa on inflammatory markers in HIV patients with excess abdominal fat. AIDS, 28(13), 1891-1901.
[3] Grunfeld, C., et al. (2010). Effects of tesa on glucose metabolism in HIV patients with lipodystrophy syndrome. Journal of Acquired Immune Deficiency Syndromes, 53(3), 311-322.
[4] Kotler, D. P., et al. (2011). Safety and efficacy of tesa in HIV patients with lipodystrophy: 26-week results. Antiviral Therapy, 16(7), 1039-1048.
[5] Mamputu, J. C., et al. (2012). Effects of tesa on glucose homeostasis in healthy volunteers and patients with type 2 diabetes. Diabetes Care, 35(8), 1584-1591.
SEO Meta Information
Meta Title: tesa Side Effects: Complete Safety Guide 2025
Meta Description: Comprehensive guide to tesa side effects, safety monitoring, and risk management. Learn about common reactions, serious complications, and safe usage protocols.
