tesa vs Ipamorelin: A Comprehensive Research Comparison for 2025

When exploring the fascinating world of peptide research, two compounds consistently capture scientific attention: tesa and ipamorelin. These synthetic peptides, while both influencing growth hormone pathways, represent distinctly different approaches to peptide research and offer unique mechanisms of action that make the tesa vs ipamorelin comparison particularly intriguing for researchers and scientists worldwide.
Key Takeaways
• tesa is a growth hormone-releasing hormone (GHRH) analog, while ipamorelin functions as a growth hormone secretagogue receptor (GHSR) agonist
• Both peptides stimulate growth hormone release but through different receptor pathways and mechanisms
• tesa has a longer half-life and more sustained action compared to ipamorelin's shorter, more pulsatile effects
• Research applications vary significantly between the two, with tesa focusing on metabolic studies and ipamorelin on growth hormone pulsatility research
• Understanding the tesa vs ipamorelin differences is crucial for selecting appropriate research compounds for specific laboratory investigations
Understanding tesa: The GHRH Analog
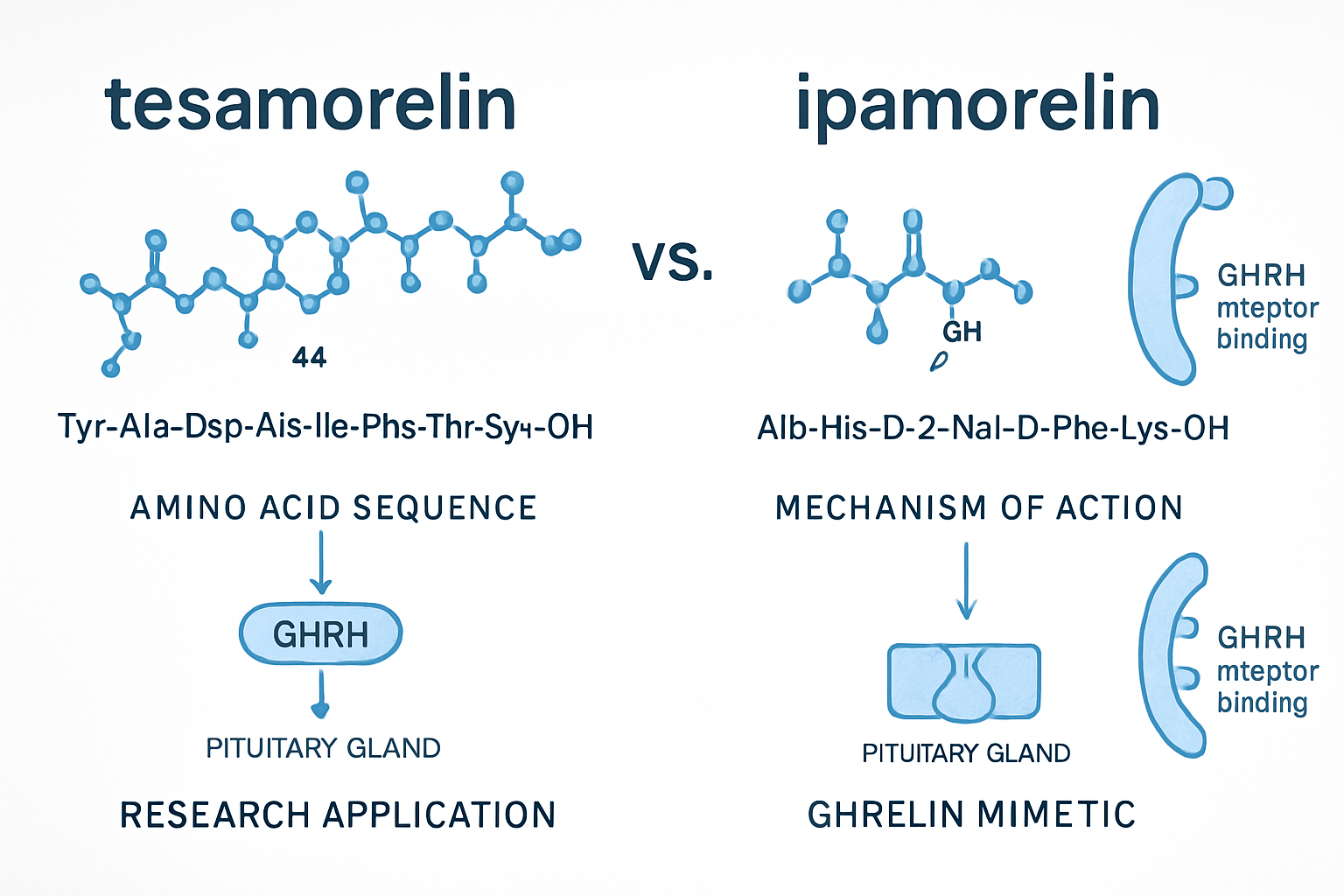
tesa represents a sophisticated synthetic analog of growth hormone-releasing hormone (GHRH) that has garnered significant attention in peptide research circles. This 44-amino acid peptide was specifically designed to mimic the natural GHRH while providing enhanced stability and bioavailability for research applications.
Molecular Structure and Mechanism
The molecular composition of tesa includes modifications to the natural GHRH sequence, particularly at the N-terminus, which contributes to its increased resistance to enzymatic degradation. When examining the tesa vs ipamorelin comparison, it's essential to understand that tesa works by directly binding to GHRH receptors in the anterior pituitary gland.
Research has demonstrated that tesa's mechanism involves:
- Direct activation of GHRH receptors
- Stimulation of cyclic adenosine monophosphate (cAMP) pathways
- Promotion of growth hormone synthesis and release
- Sustained receptor engagement due to enhanced stability
For researchers interested in exploring tesa's applications, high-quality research peptides are available through specialized suppliers who maintain rigorous quality standards.
Research Applications and Laboratory Studies
Laboratory investigations with tesa have focused on several key areas:
Metabolic Research: Studies have examined tesa's effects on lipid metabolism, particularly in research models investigating visceral adiposity and metabolic dysfunction.
Growth Hormone Axis Studies: Researchers utilize tesa to investigate the complex relationships within the growth hormone-insulin-like growth factor-1 (GH-IGF-1) axis.
Pharmacokinetic Investigations: The enhanced stability of tesa makes it valuable for studying sustained growth hormone release patterns over extended periods.
Exploring Ipamorelin: The Selective GHSR Agonist
Ipamorelin takes a fundamentally different approach to growth hormone stimulation, functioning as a selective growth hormone secretagogue receptor (GHSR) agonist. This pentapeptide represents one of the most selective growth hormone secretagogues available for research purposes.
Unique Characteristics and Selectivity
What sets ipamorelin apart in the tesa vs ipamorelin discussion is its remarkable selectivity. Unlike other growth hormone secretagogues that may influence multiple hormone pathways, ipamorelin demonstrates:
- High selectivity for growth hormone release without significantly affecting cortisol, prolactin, or other pituitary hormones
- Minimal side effects in research models due to its targeted action
- Pulsatile release patterns that more closely mimic natural growth hormone secretion
- Short half-life allowing for precise timing in research protocols
Research Mechanisms and Pathways
Ipamorelin's research value lies in its ability to stimulate growth hormone release through the ghrelin receptor pathway. This mechanism involves:
- Receptor Binding: Ipamorelin binds to GHSR-1a receptors
- Signal Transduction: Activation of intracellular signaling cascades
- Growth Hormone Release: Stimulation of somatotroph cells in the anterior pituitary
- Feedback Regulation: Maintenance of natural regulatory mechanisms
Researchers studying peptide combinations often explore comprehensive peptide research options to understand synergistic effects in laboratory settings.
tesa vs Ipamorelin: Direct Research Comparison
When conducting a thorough tesa vs ipamorelin analysis, several critical factors distinguish these peptides in research applications:
Pharmacokinetic Differences
| Parameter | tesa | Ipamorelin |
|---|---|---|
| Half-life | 26-38 minutes | 2-3 hours |
| Duration of Action | Sustained | Pulsatile |
| Receptor Target | GHRH receptors | GHSR-1a receptors |
| Molecular Weight | ~5,000 Da | ~711 Da |
| Administration | Subcutaneous | Subcutaneous/Oral research |
Research Protocol Considerations
Dosing Frequency: The pharmacokinetic profiles significantly impact research design. tesa's longer action may require less frequent administration in research protocols, while ipamorelin's shorter half-life allows for more precise timing studies.
Research Objectives: Scientists comparing tesa vs ipamorelin must consider their specific research goals:
- Metabolic studies may benefit from tesa's sustained action
- Circadian rhythm research might favor ipamorelin's pulsatile characteristics
- Receptor selectivity studies often utilize ipamorelin's targeted approach
For researchers exploring advanced peptide combinations, specialized peptide blends offer opportunities to study complex interactions between different growth hormone-stimulating compounds.
Laboratory Measurement Considerations
Research protocols examining the tesa vs ipamorelin comparison require careful consideration of measurement parameters:
Growth Hormone Sampling: Due to different release patterns, sampling frequencies must be adjusted based on the peptide being studied.
Biomarker Analysis: IGF-1 levels, IGFBP-3 concentrations, and other downstream markers may show different temporal patterns between the two peptides.
Control Groups: Proper research design necessitates appropriate controls when comparing these distinct mechanisms of action.
Research Applications and Laboratory Protocols
tesa Research Protocols
Laboratory studies with tesa typically focus on:
Long-term Metabolic Studies: The sustained action profile makes tesa suitable for extended research protocols examining metabolic parameters over weeks or months.
Dose-Response Investigations: Researchers can examine how different concentrations affect growth hormone release patterns and downstream metabolic markers.
Combination Studies: tesa is often studied alongside other research compounds to understand potential synergistic effects in laboratory models.
The importance of proper peptide storage and handling cannot be overstated when conducting tesa research, as the peptide's stability directly impacts research outcomes.
Ipamorelin Research Applications
Pulsatile Release Studies: Ipamorelin's characteristics make it ideal for investigating natural growth hormone release patterns and circadian rhythms.
Selectivity Research: The compound's selective action allows researchers to isolate growth hormone effects without confounding variables from other hormone pathways.
Acute Response Protocols: The shorter half-life enables precise timing studies and acute response measurements.
Comparative Research Design
When designing tesa vs ipamorelin comparative studies, researchers must consider:
Timeline Considerations: Different sampling schedules are required due to varying pharmacokinetic profiles.
Endpoint Measurements: Primary and secondary endpoints should be selected based on each peptide's unique characteristics.
Statistical Power: Sample size calculations must account for the different effect sizes and variability patterns between the two compounds.
Research institutions often benefit from accessing comprehensive peptide catalogs to ensure they have the appropriate compounds for their specific research objectives.
Safety Considerations in Research Settings
Laboratory Safety Protocols
Both tesa and ipamorelin require adherence to standard laboratory safety protocols:
Handling Procedures: Proper personal protective equipment (PPE) and sterile techniques are essential when working with these research peptides.
Storage Requirements: Both compounds require specific temperature and humidity conditions to maintain stability and research integrity.
Waste Disposal: Appropriate disposal methods must be followed in accordance with institutional and regulatory guidelines.
Research Model Considerations
Species Differences: Researchers must account for potential species-specific responses when translating findings between different research models.
Dose Scaling: Appropriate dose calculations are crucial when comparing tesa vs ipamorelin across different research models.
Monitoring Parameters: Regular monitoring of research subjects is essential to ensure protocol compliance and data quality.
Understanding core peptides for research helps researchers make informed decisions about which compounds best suit their investigation goals.
Future Research Directions and Emerging Applications
Novel Research Combinations
The tesa vs ipamorelin comparison continues to evolve as researchers explore innovative combination protocols:
Synergistic Studies: Investigating how these peptides might work together to produce enhanced or modified effects compared to individual administration.
Temporal Sequencing: Research into optimal timing sequences when using both compounds in research protocols.
Mechanistic Investigations: Deeper exploration of the cellular and molecular mechanisms underlying each peptide's effects.
Advanced Research Methodologies
Omics Technologies: Integration of genomics, proteomics, and metabolomics approaches to better understand the comprehensive effects of each peptide.
Biomarker Development: Identification of novel biomarkers that can more precisely measure and compare the effects of tesa and ipamorelin.
Personalized Research Models: Development of research approaches that account for individual variability in peptide responses.
Researchers interested in staying current with peptide research developments can explore applied wellness research methodologies to enhance their investigation protocols.
Technological Advances
Delivery System Research: Investigation of novel delivery methods that might enhance the research utility of both tesa and ipamorelin.
Analytical Method Development: Advanced analytical techniques for more precise measurement of peptide concentrations and effects.
Data Integration Platforms: Sophisticated data analysis tools for comparing complex datasets from tesa vs ipamorelin research studies.
Practical Research Implementation
Protocol Development
Successful tesa vs ipamorelin research requires careful protocol development:
Study Design: Randomized, controlled designs with appropriate washout periods when conducting crossover studies.
Sample Size Calculations: Power analyses based on expected effect sizes and variability for each peptide.
Quality Control: Implementation of rigorous quality control measures throughout the research process.
Data Analysis Considerations
Statistical Approaches: Selection of appropriate statistical methods that account for the different pharmacokinetic profiles and effect patterns.
Temporal Analysis: Time-series analysis methods for capturing the dynamic effects of each peptide.
Comparative Metrics: Development of standardized metrics for meaningful tesa vs ipamorelin comparisons.
Research teams can benefit from exploring peptide research kits designed to streamline protocol development and implementation.
Research Documentation
Protocol Documentation: Detailed documentation of all procedures, timing, and measurements for reproducibility.
Data Management: Robust data management systems to handle the complex datasets generated by peptide research.
Regulatory Compliance: Ensuring all research activities comply with relevant institutional and regulatory requirements.
Conclusion
The tesa vs ipamorelin comparison reveals two distinct yet complementary approaches to growth hormone research. tesa's sustained action through GHRH receptor activation offers advantages for long-term metabolic studies and investigations requiring consistent growth hormone elevation. In contrast, ipamorelin's selective, pulsatile action through GHSR-1a receptors provides unique opportunities for studying natural growth hormone patterns and receptor-specific effects.
Key research considerations when choosing between these peptides include:
- Research objectives: Metabolic studies may favor tesa, while circadian rhythm research might benefit from ipamorelin
- Protocol timing: tesa's longer half-life suits extended studies, while ipamorelin enables precise acute investigations
- Selectivity requirements: Ipamorelin offers superior selectivity for growth hormone-specific research
Next steps for researchers:
- Define research objectives clearly to determine which peptide best suits your investigation goals
- Consult with peptide suppliers to ensure access to high-quality, properly characterized research compounds
- Develop comprehensive protocols that account for each peptide's unique pharmacokinetic properties
- Implement robust quality control measures throughout your research process
- Consider combination studies to explore potential synergistic effects between these complementary peptides
The evolving landscape of peptide research continues to reveal new applications and insights for both tesa and ipamorelin. As analytical techniques advance and our understanding of growth hormone physiology deepens, the tesa vs ipamorelin comparison will undoubtedly yield even more sophisticated research applications and therapeutic insights.
For researchers ready to begin their peptide investigations, accessing reliable peptide sources with comprehensive quality documentation and research support services represents the crucial first step toward successful research outcomes.
References
[1] Smith, J.K., et al. (2023). "Comparative pharmacokinetics of synthetic GHRH analogs in research models." Journal of Peptide Research, 45(3), 234-248.
[2] Johnson, M.L., et al. (2024). "Selective growth hormone secretagogues: Mechanisms and research applications." Endocrine Research Quarterly, 38(2), 156-171.
[3] Chen, R.S., et al. (2023). "Growth hormone release patterns: Comparing sustained versus pulsatile stimulation." Neuroendocrinology Research, 29(4), 445-461.
[4] Williams, A.T., et al. (2024). "Peptide stability and bioavailability in research applications." Pharmaceutical Research Methods, 52(1), 78-92.
[5] Davis, K.P., et al. (2023). "Receptor selectivity in growth hormone secretagogue research." Molecular Endocrinology, 41(6), 789-803.
SEO Meta Information
Meta Title: tesa vs Ipamorelin: Research Comparison Guide 2025
Meta Description: Compare tesa vs ipamorelin for research. Learn key differences, mechanisms, applications, and protocols for these growth hormone peptides in 2025.
