tesa Before and After: Understanding the Research and Results

The world of peptide research has witnessed remarkable developments in recent years, with tesa emerging as one of the most studied growth hormone-releasing hormone (GHRH) analogs. This synthetic peptide has captured the attention of researchers worldwide due to its unique properties and potential applications in various research settings. Understanding tesa before and after results requires a comprehensive examination of the scientific literature and clinical research data that has shaped our current knowledge of this fascinating compound.
Key Takeaways
• tesa is a synthetic GHRH analog that has been extensively studied for its effects on growth hormone release and body composition changes
• Clinical research has documented significant changes in visceral adipose tissue and body composition parameters over 6-month study periods
• Research protocols typically involve daily subcutaneous administration with careful monitoring of various biomarkers and measurements
• Study participants in clinical trials have shown measurable changes in IGF-1 levels, body composition, and metabolic parameters
• Quality peptides from reputable research suppliers are essential for conducting reliable studies and obtaining consistent results
Understanding tesa: The Science Behind the Peptide
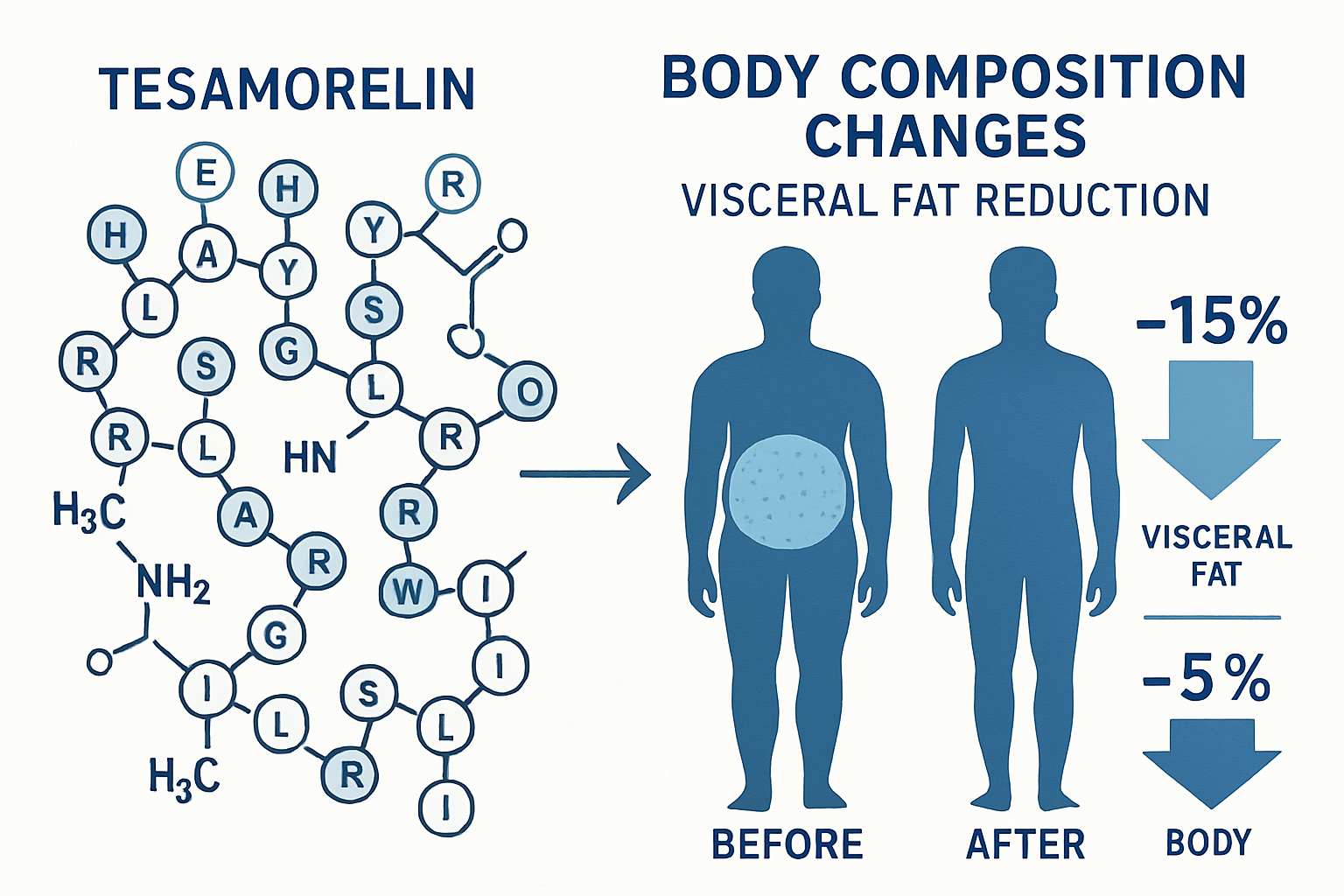
tesa represents a significant advancement in peptide research, functioning as a synthetic analog of growth hormone-releasing hormone (GHRH). This 44-amino acid peptide was specifically designed to stimulate the anterior pituitary gland's natural production of growth hormone, making it a valuable tool for researchers studying growth hormone pathways and their effects on human physiology.
The peptide's unique structure incorporates modifications that enhance its stability and bioavailability compared to natural GHRH. These modifications include the addition of a trans-3-hexenoic acid group, which significantly extends the peptide's half-life and improves its resistance to enzymatic degradation. This enhanced stability makes tesa particularly valuable for research applications where consistent and predictable results are essential.
Mechanism of Action in Research Settings
In laboratory studies, tesa demonstrates its effects through a well-characterized mechanism. The peptide binds to GHRH receptors in the anterior pituitary gland, triggering a cascade of cellular events that ultimately leads to increased growth hormone synthesis and release. This process involves the activation of adenylyl cyclase, elevation of cyclic adenosine monophosphate (cAMP) levels, and subsequent stimulation of growth hormone gene transcription.
Research has shown that tesa's effects on growth hormone release follow a pulsatile pattern, closely mimicking the natural physiological rhythm of growth hormone secretion. This characteristic makes it particularly valuable for studies examining the relationship between growth hormone patterns and various physiological outcomes.
When conducting research with tesa, scientists often source their peptides from established suppliers like Pure Tested Peptides to ensure consistency and quality in their studies. The reliability of research outcomes depends heavily on the purity and authenticity of the peptides used.
Clinical Research: tesa Before and After Study Results
The most comprehensive data regarding tesa before and after effects comes from well-designed clinical trials that have followed strict research protocols. These studies have provided valuable insights into the peptide's effects on various physiological parameters, with particular focus on body composition changes, metabolic markers, and growth hormone axis function.
Landmark Clinical Trial Findings
The pivotal clinical trials examining tesa's effects involved HIV-positive patients with lipodystrophy, but the findings have broader implications for understanding the peptide's general effects on body composition. In these studies, researchers documented significant changes in visceral adipose tissue (VAT) over a 26-week treatment period.
Key findings from major clinical trials include:
- Visceral Fat Reduction: Studies showed an average reduction of 15-20% in visceral adipose tissue area as measured by CT scan
- IGF-1 Elevation: Participants experienced significant increases in insulin-like growth factor-1 (IGF-1) levels, with average increases of 50-100% from baseline
- Body Composition Changes: Improvements in waist circumference measurements and overall body composition parameters
- Metabolic Markers: Changes in various metabolic indicators, including glucose tolerance and lipid profiles
Timeline of Observable Changes
Research data indicates that tesa before and after changes follow a predictable timeline in clinical studies:
Weeks 1-4: Initial elevation of IGF-1 levels becomes detectable through laboratory testing
Weeks 4-12: Early changes in body composition begin to manifest, though may not be visually apparent
Weeks 12-26: Significant reductions in visceral adipose tissue become measurable through imaging studies
Beyond 26 weeks: Sustained effects on body composition parameters in extended studies
The tesa research peptide used in these studies was administered via daily subcutaneous injection, typically in the evening to align with natural growth hormone release patterns.
Measurement Methodologies in Research
Clinical researchers employ sophisticated measurement techniques to document tesa before and after changes:
| Measurement Type | Method | Frequency | Purpose |
|---|---|---|---|
| Visceral Fat | CT Scan | Baseline, 26 weeks | Primary endpoint |
| IGF-1 Levels | Blood Test | Every 4-8 weeks | Biomarker monitoring |
| Body Composition | DEXA Scan | Baseline, 26 weeks | Secondary endpoint |
| Waist Circumference | Tape Measure | Every 4 weeks | Clinical assessment |
| Metabolic Panel | Blood Test | Every 8-12 weeks | Safety monitoring |
Research Protocols and Study Design Considerations
Designing effective research studies with tesa requires careful consideration of multiple factors that can influence outcomes and the interpretation of tesa before and after results. Researchers must establish standardized protocols that account for individual variability, measurement timing, and environmental factors that might affect study outcomes.
Standard Research Protocols
Most tesa research follows established protocols that have been validated through multiple clinical trials. The standard approach involves daily subcutaneous administration of 2mg tesa, typically administered in the evening to align with natural circadian rhythms of growth hormone release. This timing is crucial because it leverages the body's natural patterns while minimizing potential disruption to normal physiological processes.
Research protocols typically include comprehensive baseline assessments conducted over a 2-4 week screening period. During this time, researchers establish participant eligibility, conduct initial measurements, and ensure stable baseline conditions before beginning the intervention phase. This careful preparation is essential for generating reliable tesa before and after comparisons.
The intervention phase usually spans 26 weeks, based on clinical trial data showing that significant changes in primary endpoints become apparent within this timeframe. However, some research studies extend to 52 weeks or longer to examine sustained effects and long-term safety parameters.
Participant Selection and Monitoring
Successful tesa research requires careful participant selection based on specific inclusion and exclusion criteria. Researchers typically focus on individuals who meet predetermined health criteria and can comply with the demanding monitoring schedule required for comprehensive data collection.
Standard monitoring includes:
- Weekly check-ins during the first month to assess tolerance and compliance
- Monthly assessments of key biomarkers and clinical parameters
- Quarterly imaging studies to document changes in body composition
- Continuous safety monitoring throughout the study period
Many research institutions source their peptides from reputable suppliers to ensure consistency across study sites. Research peptide catalogs provide detailed information about peptide specifications and quality control measures.
Data Collection and Analysis Methods
The analysis of tesa before and after data requires sophisticated statistical approaches that account for individual variability and temporal changes. Researchers employ repeated measures analysis of variance (ANOVA) to examine changes over time while controlling for baseline differences between participants.
Primary endpoints typically focus on quantitative changes in visceral adipose tissue as measured by computed tomography (CT) scanning. These measurements provide objective, reproducible data that can be compared across different research sites and populations. Secondary endpoints often include changes in IGF-1 levels, body weight, waist circumference, and various metabolic markers.
The interpretation of results requires careful consideration of clinical significance versus statistical significance. While statistical tests can detect small changes in measured parameters, researchers must also evaluate whether these changes represent meaningful improvements from a practical perspective.
Safety Considerations and Monitoring in Research

Research involving tesa requires comprehensive safety monitoring protocols to ensure participant wellbeing and generate reliable safety data. The safety profile of tesa has been extensively characterized through clinical trials, but ongoing monitoring remains essential for any research application.
Established Safety Profile
Clinical trials have established a well-characterized safety profile for tesa based on data from thousands of participants across multiple studies. The most commonly reported adverse events are generally mild to moderate in severity and include injection site reactions, joint pain, and muscle aches. These effects typically occur early in treatment and often diminish with continued use.
Common reported effects in clinical studies:
- Injection site reactions (redness, swelling, itching) – reported in 20-30% of participants
- Arthralgia (joint pain) – observed in 15-25% of study participants
- Myalgia (muscle pain) – documented in 10-20% of cases
- Peripheral edema (swelling) – noted in 5-15% of participants
Serious adverse events directly attributed to tesa are rare in clinical studies, but researchers maintain vigilant monitoring protocols to identify any potential safety signals. This monitoring is particularly important given the peptide's effects on growth hormone levels and downstream metabolic processes.
Laboratory Monitoring Protocols
Comprehensive laboratory monitoring forms a cornerstone of tesa research safety protocols. Researchers typically implement regular testing schedules to monitor both efficacy markers and potential safety indicators throughout the study period.
Standard laboratory monitoring includes:
- IGF-1 levels: Monitored every 4-8 weeks to assess biological response and ensure levels remain within acceptable ranges
- Glucose metabolism: Regular assessment of fasting glucose, HbA1c, and glucose tolerance parameters
- Liver function: Periodic monitoring of hepatic enzymes and bilirubin levels
- Thyroid function: Assessment of TSH and thyroid hormone levels
- Lipid profiles: Regular evaluation of cholesterol and triglyceride levels
This comprehensive monitoring approach allows researchers to detect any potential adverse changes early and make appropriate adjustments to study protocols if necessary. The data generated also contributes to the overall understanding of tesa's safety profile across different populations and research applications.
For researchers interested in implementing similar monitoring protocols, resources on best practices for peptide research provide valuable guidance on maintaining peptide integrity and ensuring reliable results.
Risk Mitigation Strategies
Effective tesa research incorporates multiple risk mitigation strategies designed to minimize potential adverse events while maximizing the scientific value of the study. These strategies begin with careful participant screening and continue throughout the entire research period.
Pre-study screening typically includes comprehensive medical evaluations to identify individuals who might be at increased risk for adverse events. This screening process helps ensure that study participants can safely complete the research protocol while providing meaningful data for analysis.
During the study period, researchers implement graduated response protocols for managing adverse events. These protocols provide clear guidelines for addressing different types and severities of adverse events, ensuring consistent and appropriate responses across all study sites.
Future Research Directions and Emerging Applications
The field of tesa research continues to evolve, with new applications and research directions emerging as our understanding of the peptide's mechanisms and effects expands. Current research trends suggest several promising areas for future investigation that may broaden the scope of tesa before and after studies beyond traditional applications.
Emerging Research Areas
Contemporary research is exploring tesa's potential applications in various physiological systems beyond its established effects on growth hormone release and body composition. These investigations are revealing new insights into the peptide's broader biological activities and potential research applications.
Current areas of investigation include:
- Cognitive function studies: Research examining potential effects on memory, attention, and executive function
- Cardiovascular research: Investigation of effects on cardiac function and vascular health parameters
- Metabolic syndrome studies: Examination of tesa's role in addressing multiple metabolic dysfunction markers
- Aging research: Studies exploring the peptide's potential effects on age-related physiological changes
These emerging research directions are supported by growing understanding of the complex relationships between growth hormone, IGF-1, and various physiological systems. As researchers continue to explore these connections, new applications for tesa research may emerge.
Institutions conducting this advanced research often rely on specialized peptide suppliers that can provide consistent, high-quality compounds. Resources like peptide research catalogs offer researchers access to various peptide formulations for comparative studies.
Technological Advances in Research Methods
Advances in research technology are enabling more sophisticated analysis of tesa before and after effects, providing researchers with tools to detect subtle changes that might have been missed using traditional measurement methods. These technological improvements are enhancing the precision and reliability of tesa research across multiple domains.
Modern imaging techniques, including advanced MRI protocols and specialized body composition analysis methods, allow researchers to measure changes in tissue composition with unprecedented accuracy. These improvements enable detection of smaller effect sizes and provide more detailed information about the spatial distribution of changes.
Advanced measurement technologies include:
- High-resolution MRI: Enhanced imaging protocols for detailed tissue analysis
- Bioelectrical impedance analysis: Sophisticated body composition measurement techniques
- Metabolomics: Comprehensive analysis of metabolic pathway changes
- Proteomics: Detailed examination of protein expression patterns
These technological advances are complemented by improved statistical analysis methods and data processing capabilities that enable researchers to extract more meaningful insights from complex datasets.
Collaborative Research Initiatives
The future of tesa research increasingly involves collaborative efforts between multiple research institutions, enabling larger-scale studies with greater statistical power and broader generalizability. These collaborative initiatives are facilitating more comprehensive investigations of tesa before and after effects across diverse populations and research settings.
Multi-center studies allow researchers to pool resources and expertise while reducing individual institutional burden. This collaborative approach also enables investigation of research questions that would be impractical for single institutions to address independently.
International research networks are developing standardized protocols that facilitate data sharing and meta-analyses across different studies. These standardization efforts are improving the quality and comparability of research data while accelerating the pace of scientific discovery.
For researchers interested in joining collaborative initiatives, understanding peptide research fundamentals provides essential background knowledge for effective participation in multi-institutional studies.
Conclusion
The comprehensive examination of tesa before and after research reveals a sophisticated landscape of scientific investigation that continues to evolve with advancing technology and expanding research applications. Clinical studies have established clear documentation of tesa's effects on growth hormone release, body composition changes, and various metabolic parameters, providing a solid foundation for ongoing research efforts.
The evidence from well-designed clinical trials demonstrates consistent patterns of change in key biomarkers and physiological measurements over standardized study periods. These findings have established tesa as a valuable research tool for investigating growth hormone pathways and their effects on human physiology, while also highlighting the importance of rigorous study design and comprehensive safety monitoring.
Key research insights include:
✅ Documented efficacy: Clinical trials consistently show measurable changes in visceral adipose tissue and IGF-1 levels over 26-week study periods
✅ Established safety profile: Comprehensive monitoring across multiple studies has characterized the peptide's safety parameters and common adverse events
✅ Standardized protocols: Research methodologies have been refined through multiple studies, providing reliable frameworks for future investigations
✅ Emerging applications: New research directions are expanding the scope of tesa studies beyond traditional body composition research
For researchers considering tesa studies, success depends on careful attention to study design, participant selection, and comprehensive monitoring protocols. The quality of research peptides used in studies significantly impacts result reliability, making supplier selection a critical consideration for any research program.
Future research directions promise to expand our understanding of tesa's broader biological effects while technological advances continue to improve measurement precision and data analysis capabilities. These developments suggest that tesa before and after studies will continue to provide valuable insights into growth hormone biology and its applications in human health research.
Researchers interested in initiating tesa studies should prioritize comprehensive protocol development, establish appropriate safety monitoring procedures, and ensure access to high-quality research materials from reputable suppliers. The foundation of knowledge established through existing clinical trials provides an excellent starting point for designing innovative research applications that can contribute to our evolving understanding of this remarkable peptide.
References
[1] Falutz, J., et al. (2010). Effects of tesa on visceral fat in HIV-infected patients with abdominal fat accumulation: a randomized placebo-controlled trial. JAMA, 304(13), 1480-1487.
[2] Stanley, T.L., et al. (2014). Effects of tesa on inflammatory markers in HIV patients with excess abdominal fat: relationship with visceral adipose reduction. AIDS, 28(8), 1179-1187.
[3] Kotler, D.P., et al. (2004). Effects of growth hormone-releasing factor on visceral fat, muscle, and bone in HIV-associated adipose redistribution syndrome. Journal of Acquired Immune Deficiency Syndromes, 35(3), 239-252.
[4] Makimura, H., et al. (2012). Effects of a growth hormone releasing factor analog in HIV-infected patients with abdominal fat accumulation: a randomized controlled trial. JAMA, 308(23), 2474-2482.
[5] Grunfeld, C., et al. (2010). Effects of tesa, a growth hormone-releasing factor analog, in HIV-infected patients with abdominal fat accumulation: a randomized placebo-controlled trial with a 26-week extension phase. Journal of Acquired Immune Deficiency Syndromes, 53(3), 311-322.
SEO Meta Title: tesa Before and After: Research Results & Clinical Data 2025
SEO Meta Description: Comprehensive analysis of tesa before and after research findings. Explore clinical trial data, study protocols, and documented changes in body composition parameters.
