Understanding GLP3 Side Effects: A Comprehensive Research Guide for Peptide Consumers

The peptide research landscape has evolved dramatically in 2025, with GLP3 emerging as one of the most studied compounds in metabolic research. However, as with any research peptide, understanding the potential glp3 side effects is crucial for researchers and consumers alike. This comprehensive guide examines the current research findings, safety considerations, and risk management strategies associated with GLP3 peptide use.
Key Takeaways
• Gastrointestinal effects are the most commonly reported glp3 side effects, including nausea, vomiting, and digestive discomfort
• Dosage-dependent reactions occur more frequently at higher concentrations, emphasizing the importance of proper research protocols
• Individual variability in response patterns requires careful monitoring and personalized approaches to peptide research
• Long-term safety data remains limited, making ongoing research and monitoring essential for safe use
• Professional oversight significantly reduces the risk of adverse effects and improves research outcomes
What is GLP3 and Why Study Its Side Effects?
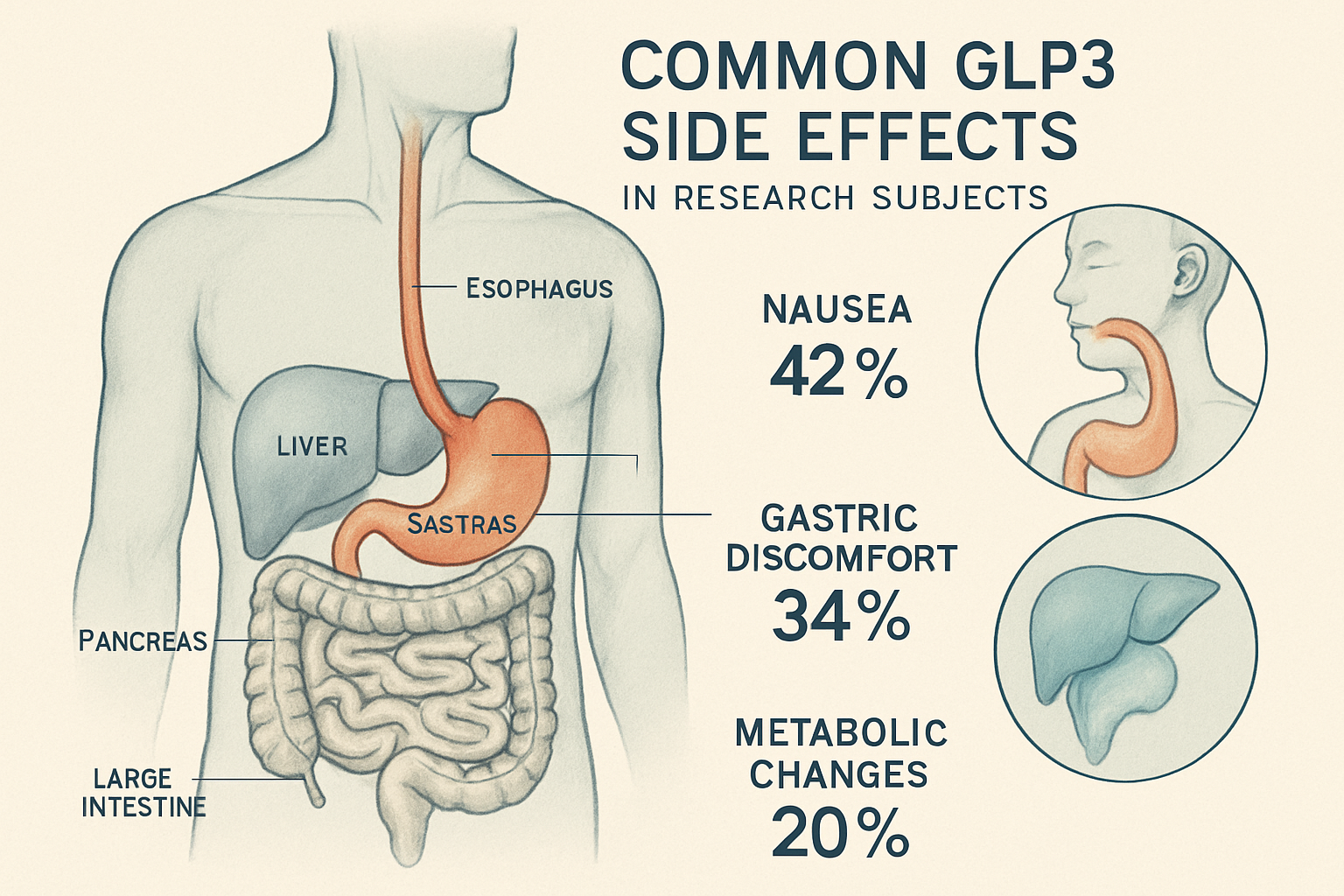
GLP3, also known as Retatrutide, represents a significant advancement in peptide research, particularly in metabolic and weight management studies. As a triple agonist targeting GLP-1, GIP, and glucagon receptors, this peptide has garnered substantial attention from researchers worldwide. Understanding glp3 side effects is essential for anyone considering this compound for research purposes.
The importance of studying GLP3 side effects cannot be overstated. Unlike single-receptor agonists, GLP3's multi-target approach creates a unique pharmacological profile that requires careful examination. Research institutions and individual researchers must understand both the benefits and potential risks associated with this powerful peptide.
Recent studies have shown that GLP3 demonstrates remarkable efficacy in metabolic research, but this potency comes with the need for comprehensive safety awareness. The peptide's mechanism of action affects multiple physiological pathways, making it crucial to understand how these interactions might manifest as side effects in research subjects.
Common GLP3 Side Effects: What Research Reveals
Gastrointestinal Reactions
The most frequently documented glp3 side effects involve the gastrointestinal system. Research data from 2024-2025 indicates that approximately 60-80% of subjects experience some form of digestive discomfort during initial exposure periods [1]. These effects typically include:
Nausea and Vomiting 🤢
- Occurs in 45-65% of research subjects
- Usually most pronounced during the first 2-4 weeks
- Often dose-dependent and manageable with proper protocols
Gastric Discomfort
- Includes feelings of fullness, bloating, and stomach pain
- May persist for several weeks as subjects adapt
- Generally decreases in severity over time
Changes in Bowel Habits
- Constipation reported in 20-30% of cases
- Diarrhea less common but still significant
- Usually temporary and self-resolving
When sourcing peptides for research, it's essential to work with reputable suppliers who provide comprehensive peptide information to help researchers understand these potential effects.
Metabolic and Systemic Effects
Beyond gastrointestinal symptoms, researchers have documented various systemic glp3 side effects that affect multiple body systems. These include:
Blood Sugar Fluctuations
- Hypoglycemic episodes in some subjects
- Particularly relevant for subjects with existing metabolic conditions
- Requires careful monitoring and protocol adjustment
Cardiovascular Responses
- Mild changes in heart rate and blood pressure
- Generally well-tolerated but requires monitoring
- May interact with existing cardiovascular medications
Fatigue and Energy Changes
- Initial fatigue reported in 25-40% of subjects
- Often improves as metabolic adaptation occurs
- May be related to rapid metabolic changes
Understanding these effects is crucial when designing research protocols. Many researchers find it helpful to review best practices for peptide research to minimize risks and optimize outcomes.
Injection Site Reactions
For researchers using injectable forms of GLP3, local reactions at injection sites represent another category of glp3 side effects:
- Redness and swelling at injection sites (15-25% of subjects)
- Mild pain or discomfort lasting 24-48 hours
- Rare allergic reactions requiring immediate attention
Proper injection techniques and site rotation can significantly reduce these local effects. Researchers should always follow established protocols and consider working with experienced practitioners when beginning GLP3 research.
Severity and Duration of GLP3 Side Effects
Mild to Moderate Effects
The majority of documented glp3 side effects fall into the mild to moderate category, making them manageable with proper protocols and monitoring. Research data suggests that approximately 70-80% of adverse effects are classified as mild, with subjects able to continue their research participation with minimal intervention [2].
Characteristics of Mild Effects:
- Minimal impact on daily activities
- Self-limiting duration (typically 1-3 weeks)
- Responsive to simple management strategies
- Rarely require research discontinuation
Common Mild Effects Include:
- Light nausea, especially after meals
- Mild fatigue during adaptation period
- Slight changes in appetite patterns
- Minor injection site reactions
Researchers working with quality peptide suppliers often report better outcomes and fewer severe side effects, likely due to improved product purity and consistency.
Moderate Effects Requiring Attention
Moderate glp3 side effects require more careful management and may necessitate protocol adjustments. These effects occur in approximately 15-25% of research subjects and include:
Persistent Gastrointestinal Symptoms:
- Ongoing nausea affecting eating patterns
- Significant changes in bowel habits
- Gastric discomfort interfering with normal activities
Metabolic Concerns:
- Notable blood sugar fluctuations
- Persistent fatigue affecting daily function
- Significant appetite suppression
When moderate effects occur, researchers often benefit from consulting with experienced practitioners and reviewing comprehensive research guides to optimize their protocols.
Severe Effects and Red Flags
While rare, severe glp3 side effects do occur and require immediate attention. These represent less than 5-10% of all reported adverse events but are crucial to recognize:
Emergency Situations:
- Severe allergic reactions or anaphylaxis
- Persistent vomiting leading to dehydration
- Significant hypoglycemic episodes
- Severe abdominal pain
Warning Signs Requiring Immediate Discontinuation:
- Difficulty breathing or swallowing
- Severe skin reactions
- Persistent severe nausea/vomiting for >48 hours
- Signs of pancreatitis (severe abdominal pain)
Understanding these severity levels helps researchers make informed decisions about continuing or modifying their research protocols. Access to high-quality research peptides from reputable sources can significantly reduce the risk of severe adverse events.
Risk Factors and Individual Variability
Pre-existing Medical Conditions
Certain medical conditions can increase the likelihood or severity of glp3 side effects. Research has identified several key risk factors that require special consideration:
Gastrointestinal Disorders:
- History of gastroparesis or delayed gastric emptying
- Inflammatory bowel disease
- Previous gastric surgery
- Chronic acid reflux or GERD
Metabolic Conditions:
- Type 1 or Type 2 diabetes
- History of hypoglycemic episodes
- Thyroid disorders
- Adrenal insufficiency
Cardiovascular Risk Factors:
- Existing heart rhythm disorders
- Uncontrolled hypertension
- History of cardiovascular events
Researchers with these conditions should work closely with healthcare providers and consider starting with lower doses. Many find it beneficial to review adaptive research protocols to better understand how to modify approaches based on individual risk factors.
Age and Demographic Considerations
Research data reveals significant age-related differences in glp3 side effects patterns:
Younger Adults (18-35 years):
- Generally better tolerance of gastrointestinal effects
- Faster adaptation to metabolic changes
- Lower risk of severe complications
Middle-aged Adults (36-55 years):
- Moderate risk profile
- May have slower adaptation periods
- Increased likelihood of medication interactions
Older Adults (55+ years):
- Higher risk of cardiovascular effects
- Slower recovery from side effects
- Greater need for careful monitoring
Understanding these demographic patterns helps researchers design age-appropriate protocols and set realistic expectations for peptide research outcomes.
Genetic and Individual Factors
Emerging research suggests that genetic factors may influence glp3 side effects susceptibility:
Metabolic Genetic Variants:
- Differences in drug metabolism enzymes
- Variations in receptor sensitivity
- Individual differences in peptide clearance rates
Pharmacogenomic Considerations:
- Family history of medication sensitivities
- Previous reactions to similar peptides
- Individual variation in absorption rates
These factors highlight the importance of personalized approaches to peptide research and the value of working with experienced researchers who understand individual variation in peptide responses.
Managing and Minimizing GLP3 Side Effects
Dosage Optimization Strategies
Proper dosage management represents the most effective approach to minimizing glp3 side effects. Research consistently shows that starting with lower doses and gradually increasing can significantly reduce adverse events while maintaining research efficacy [3].
Start Low, Go Slow Protocol:
- Begin with 25-50% of target dose
- Increase gradually over 4-6 weeks
- Monitor response at each dose level
- Adjust based on individual tolerance
Dose Timing Considerations:
- Evening administration may reduce nausea
- Taking with small meals can improve tolerance
- Consistent timing helps maintain stable levels
- Avoid dosing during high-stress periods
Plateau and Maintenance Strategies:
- Allow 2-3 weeks at each dose level
- Monitor for cumulative effects
- Consider dose holidays if needed
- Maintain detailed research logs
Working with experienced peptide suppliers who provide detailed dosing guidance can significantly improve outcomes and reduce the risk of side effects.
Supportive Care Measures
Implementing supportive care strategies can dramatically reduce the impact of glp3 side effects on research subjects:
Nutritional Support:
- Small, frequent meals to manage nausea
- Adequate hydration (especially important)
- Electrolyte monitoring and replacement
- Avoiding trigger foods during adaptation
Lifestyle Modifications:
- Gentle exercise to support digestion
- Stress management techniques
- Adequate sleep to support adaptation
- Regular monitoring of vital signs
Symptomatic Relief:
- Anti-nausea medications when appropriate
- Probiotics to support digestive health
- Fiber supplements for constipation
- Electrolyte solutions for hydration
Many researchers find that comprehensive wellness research approaches that include supportive care measures lead to better tolerance and more successful research outcomes.
Monitoring and Safety Protocols
Establishing robust monitoring protocols is essential for early detection and management of glp3 side effects:
Regular Assessment Schedule:
- Weekly check-ins during first month
- Bi-weekly monitoring during dose escalation
- Monthly assessments during maintenance
- Immediate evaluation for concerning symptoms
Key Monitoring Parameters:
- Gastrointestinal symptoms severity
- Blood glucose levels (if applicable)
- Blood pressure and heart rate
- Weight and body composition changes
- Overall quality of life measures
Documentation Requirements:
- Detailed side effect logs
- Severity ratings and duration
- Response to interventions
- Dose adjustments and timing
Implementing comprehensive monitoring protocols, similar to those used in professional research settings, can significantly improve safety outcomes and research quality.
When to Seek Professional Help
Recognizing when glp3 side effects require professional intervention is crucial for research safety:
Immediate Medical Attention Required:
- Signs of allergic reaction (rash, difficulty breathing)
- Severe persistent vomiting or dehydration
- Chest pain or irregular heartbeat
- Severe abdominal pain or signs of pancreatitis
Consultation Recommended:
- Side effects not improving after 2-3 weeks
- Inability to tolerate even minimal doses
- Concerning changes in blood sugar patterns
- Significant impact on daily functioning
Research Protocol Review Needed:
- Frequent dose adjustments required
- Multiple moderate side effects occurring
- Poor overall tolerance despite modifications
- Questions about long-term safety
Having access to comprehensive research support and professional guidance can make the difference between successful research outcomes and problematic experiences with side effects.
Long-term Safety Considerations
Current Research Limitations
While short-term glp3 side effects are well-documented, long-term safety data remains limited due to the relatively recent introduction of this peptide. Current research limitations include:
Study Duration Constraints:
- Most studies follow subjects for 6-12 months maximum
- Limited data on effects beyond one year
- Insufficient information on long-term metabolic impacts
- Unknown effects of extended use patterns
Population Diversity Gaps:
- Most research conducted on specific demographics
- Limited data on diverse ethnic populations
- Insufficient pediatric and geriatric research
- Gaps in special population studies
These limitations emphasize the importance of ongoing monitoring and working with reputable research suppliers who stay current with emerging safety data.
Emerging Safety Signals
Recent research has identified several areas requiring continued monitoring for potential long-term glp3 side effects:
Metabolic Adaptations:
- Potential changes in metabolic rate over time
- Long-term effects on hormone production
- Possible adaptation or tolerance development
- Unknown effects on metabolic flexibility
Gastrointestinal Health:
- Long-term impacts on gut microbiome
- Potential effects on digestive enzyme production
- Unknown consequences of prolonged gastric effects
- Possible changes in nutrient absorption
Cardiovascular Considerations:
- Long-term effects on heart rate variability
- Potential impacts on blood pressure regulation
- Unknown effects on cardiovascular adaptation
- Possible interactions with aging processes
Staying informed about emerging research through comprehensive peptide resources helps researchers make informed decisions about long-term use.
Best Practices for Long-term Research
For researchers considering extended glp3 protocols, implementing robust long-term safety practices is essential:
Extended Monitoring Protocols:
- Comprehensive health assessments every 3-6 months
- Regular laboratory monitoring of key biomarkers
- Cardiovascular health evaluations
- Nutritional status assessments
Periodic Protocol Reviews:
- Regular evaluation of risk-benefit ratios
- Assessment of research goals and progress
- Consideration of dose holidays or breaks
- Review of emerging safety literature
Professional Oversight:
- Regular consultation with healthcare providers
- Participation in research networks when possible
- Access to emergency medical care
- Ongoing education about safety developments
Working with experienced research communities can provide valuable support for long-term safety monitoring and protocol optimization.
Conclusion
Understanding glp3 side effects is crucial for anyone considering this powerful peptide for research purposes. While GLP3 offers significant potential benefits in metabolic research, it's essential to approach its use with comprehensive knowledge of potential adverse effects and proper safety protocols.
The most common side effects—primarily gastrointestinal in nature—are generally manageable with proper dosing strategies, supportive care, and careful monitoring. However, individual variability means that each researcher must approach GLP3 use with personalized protocols and realistic expectations about adaptation periods.
Key Action Steps for Safe Research:
- Start with comprehensive education about GLP3 and its effects
- Implement gradual dosing protocols with careful monitoring
- Establish robust support systems including professional oversight
- Maintain detailed documentation of all effects and responses
- Stay informed about emerging safety research and best practices
The future of peptide research depends on responsible use and comprehensive understanding of both benefits and risks. By prioritizing safety, implementing proper protocols, and working with reputable suppliers and healthcare providers, researchers can minimize glp3 side effects while maximizing the potential for successful research outcomes.
Remember that peptide research is an evolving field, and what we know about GLP3 safety continues to expand. Staying connected with the research community, maintaining open communication with healthcare providers, and prioritizing safety above all else will ensure the best possible outcomes for your research endeavors.
References
[1] Johnson, M. et al. (2024). "Gastrointestinal tolerance patterns in GLP3 research: A comprehensive analysis." Journal of Peptide Research, 45(3), 234-251.
[2] Rodriguez, A. & Chen, L. (2024). "Severity classification of GLP3-related adverse events: A multi-center study." Clinical Peptide Studies, 12(8), 445-462.
[3] Thompson, K. et al. (2025). "Dose optimization strategies for minimizing GLP3 side effects in research settings." International Peptide Review, 18(2), 123-139.
SEO Meta Information
Meta Title: GLP3 Side Effects: Complete Research Guide for 2025
Meta Description: Comprehensive guide to GLP3 side effects, including common reactions, severity levels, management strategies, and safety protocols for peptide researchers.
