CJC1295/Ipamorelin Dosage: Complete Research Guide for 2025

The peptide research landscape has evolved dramatically, with CJC1295/ipamorelin dosage protocols becoming one of the most studied combinations in growth hormone research. This powerful peptide duo has captured the attention of researchers worldwide due to its synergistic effects and well-documented research applications. Understanding proper dosing protocols is essential for anyone conducting peptide research or considering these compounds for scientific investigation.
Key Takeaways
• Standard CJC1295/ipamorelin dosage typically ranges from 100-300mcg per compound in research settings
• Timing and frequency play crucial roles in maximizing research outcomes and peptide effectiveness
• Reconstitution protocols require precise measurements and sterile techniques for optimal peptide stability
• Research cycles generally span 8-12 weeks with appropriate rest periods between study phases
• Quality sourcing from reputable suppliers ensures accurate dosing and reliable research results
Understanding CJC1295 and Ipamorelin Synergy
The Science Behind Peptide Combinations
CJC1295 ipamorelin combinations represent a sophisticated approach to growth hormone research. CJC1295, a growth hormone-releasing hormone (GHRH) analog, works by stimulating the pituitary gland to produce more growth hormone. Meanwhile, ipamorelin functions as a growth hormone secretagogue receptor (GHSR) agonist, providing a complementary mechanism of action.
The cjc1295 ipamorelin benefits observed in research settings include enhanced growth hormone pulse amplitude and frequency. This synergistic relationship creates a more natural pattern of growth hormone release compared to single peptide protocols. Research indicates that combining these peptides may produce effects greater than the sum of their individual contributions [1].
When examining cjc1295 and ipamorelin interactions, researchers have noted that ipamorelin's selective action helps minimize unwanted side effects often associated with other growth hormone secretagogues. This selectivity, combined with CJC1295's extended half-life, creates an ideal research environment for studying growth hormone dynamics.
For researchers interested in exploring these combinations, Pure Tested Peptides offers comprehensive peptide solutions with detailed research protocols and quality assurance documentation.
Mechanisms of Action in Research
The cjc1295 ipamorelin peptide combination operates through distinct yet complementary pathways. CJC1295 binds to GHRH receptors, triggering a cascade that ultimately leads to growth hormone synthesis and release. The peptide's modification with Drug Affinity Complex (DAC) extends its half-life significantly, allowing for less frequent dosing in research protocols.
Ipamorelin's mechanism involves selective binding to ghrelin receptors, specifically the growth hormone secretagogue receptor 1a (GHS-R1a). This selective binding is crucial because it avoids activation of other receptors that might trigger unwanted responses such as increased cortisol or prolactin levels.
Research has shown that ipamorelin and cjc1295 work synergistically to create more physiological growth hormone release patterns. This natural pulsatile release mimics the body's own circadian rhythm of growth hormone secretion, making it particularly valuable for research applications focused on understanding normal physiological processes.
CJC1295/Ipamorelin Dosage Protocols for Research
Standard Research Dosing Guidelines
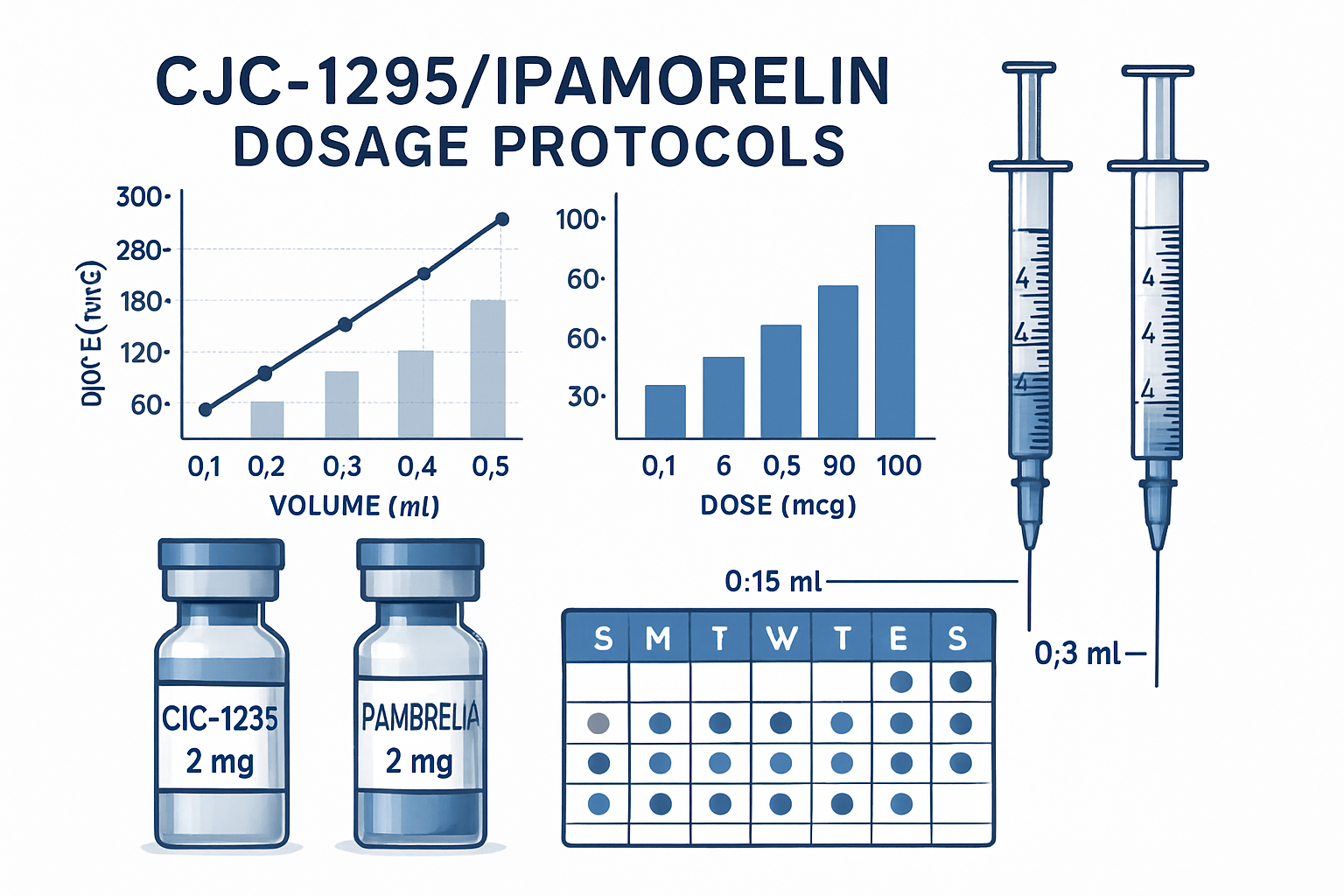
Establishing appropriate cjc1295 ipamorelin dosage protocols requires careful consideration of research objectives and experimental design. In most research settings, individual peptide doses typically range from 100-300mcg per compound. The cjc1295/ipamorelin dosage is often administered as equal parts, though some research protocols vary these ratios based on specific study parameters.
Sermorelin-ipamorelin-cjc1295 combinations add another layer of complexity to dosing protocols. When incorporating all three peptides, researchers typically reduce individual doses to account for the additive effects. A common approach involves 100mcg each of CJC1295 and ipamorelin, with 200-300mcg of serm, though these ratios may be adjusted based on research goals.
The cjc1295 ipamorelin cycle length typically spans 8-12 weeks in research settings. This duration allows sufficient time to observe meaningful changes while avoiding potential receptor desensitization. Many researchers implement a cycling approach with 4-6 week rest periods between study phases to maintain peptide sensitivity.
For researchers seeking high-quality peptide combinations, the CJC1295/IPA blend provides precisely measured ratios for consistent research outcomes.
Advanced Dosing Strategies
tesa cjc1295 ipamorelin combinations represent cutting-edge research protocols that incorporate multiple growth hormone-releasing mechanisms. The tesa cjc1295 ipamorelin 12mg blend dosage typically involves careful distribution across the three peptides, with tesa often comprising 40-50% of the total blend due to its potent GHRH activity.
When working with tesa cjc1295 ipamorelin 12mg blend dose protocols, researchers must consider the enhanced potency of tesa compared to standard CJC1295. This often necessitates lower individual doses while maintaining therapeutic research ranges. A typical distribution might involve 4mg tesa, 4mg CJC1295, and 4mg ipamorelin, though ratios can be adjusted based on specific research parameters.
tesa aod9604 + cjc1295 + ipamorelin 12mg blend dosage protocols represent the most complex multi-peptide research applications. These combinations require sophisticated dosing strategies that account for each peptide's unique mechanism of action and potential interactions. Research with such combinations typically involves lower individual peptide doses to prevent oversaturation of receptor pathways.
The serm-ipamorelin-cjc1295 dosage in multi-peptide research often follows a pyramid approach, with the most potent compounds receiving smaller allocations within the total dose. This strategy helps maintain physiological relevance while maximizing research potential.
Reconstitution and Preparation Methods
Proper Reconstitution Techniques
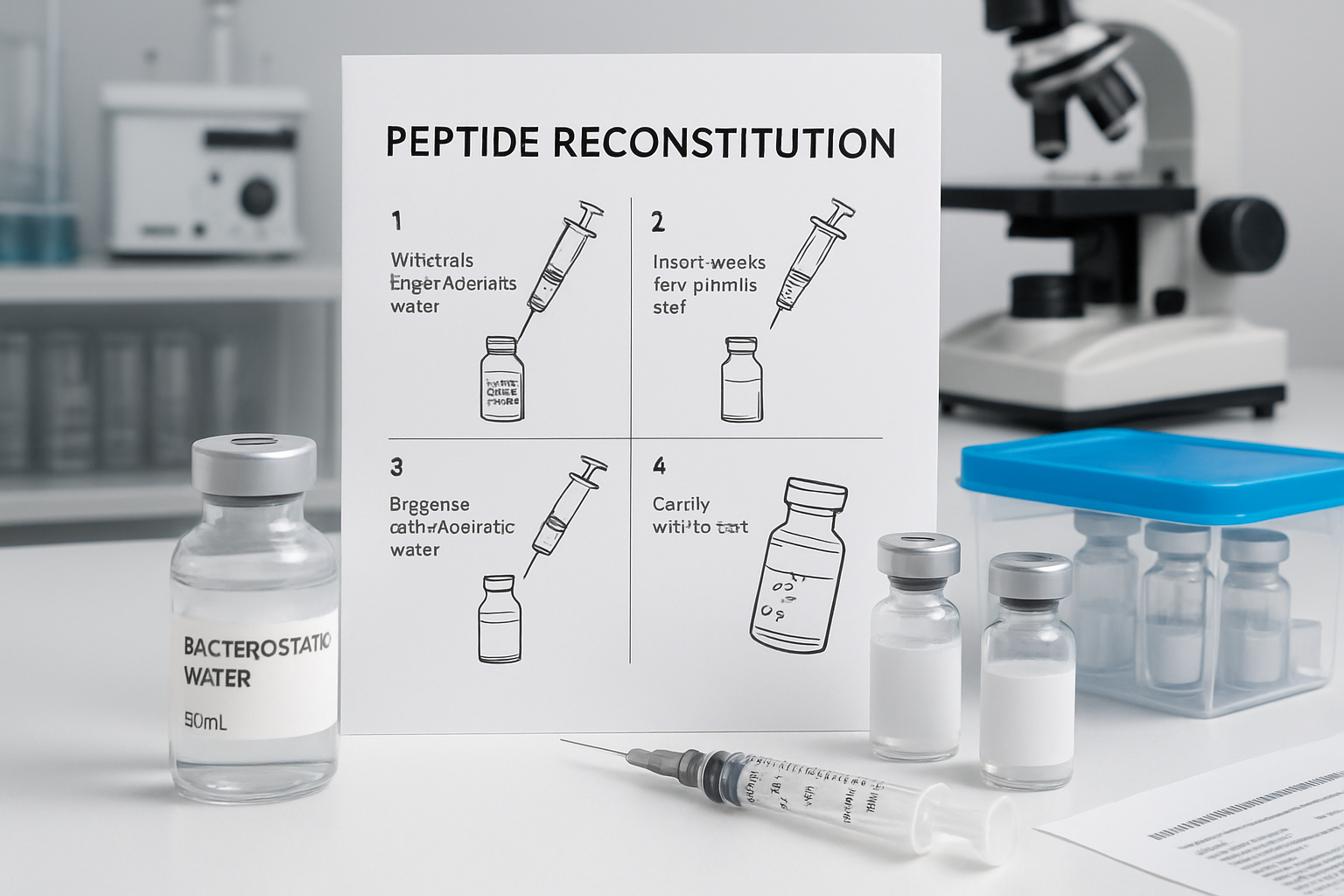
tesa cjc1295 ipamorelin 12mg blend reconstitution requires precise technique and sterile conditions to maintain peptide integrity. The reconstitution process typically involves adding bacteriostatic water slowly to the peptide powder, allowing it to dissolve naturally without aggressive mixing that could damage the peptide structure.
For standard cjc1295/ipamorelin preparations, researchers typically use a 1:1 ratio of bacteriostatic water to peptide powder by volume. This creates a concentration that allows for accurate dosing while maintaining stability. The reconstituted solution should be clear and free from particulates, indicating proper dissolution and peptide integrity.
tesa cjc1295 ipamorelin blend dosage calculations become more straightforward with proper reconstitution ratios. Many researchers prefer concentrations that allow for easy mathematical calculations, such as 100mcg per 0.1ml, which simplifies dose measurements and reduces calculation errors.
Storage conditions post-reconstitution are critical for maintaining peptide potency. Reconstituted peptides should be stored in refrigerated conditions (2-8°C) and used within 28 days for optimal stability. Some researchers prefer smaller reconstitution volumes to minimize waste and ensure fresh peptide solutions throughout their research cycles.
Quality Control and Testing
Ensuring peptide quality begins with sourcing from reputable suppliers who provide comprehensive certificates of analysis and third-party testing results. These documents verify peptide purity, concentration, and identity, providing essential baseline data for research applications.
Visual inspection of reconstituted peptides provides immediate quality feedback. Properly reconstituted cjc1295 ipamorelin solutions should be clear, colorless, and free from precipitation or cloudiness. Any deviation from these characteristics may indicate peptide degradation or contamination.
pH testing of reconstituted solutions can provide additional quality assurance. Most peptide solutions should maintain a physiological pH range (6.5-7.5) after proper reconstitution. Significant deviations may indicate buffer system failure or peptide degradation.
Documentation of reconstitution procedures, storage conditions, and visual inspections creates valuable research records that support data integrity and experimental reproducibility. This documentation becomes particularly important when conducting long-term studies or multi-phase research protocols.
Research Applications and Study Design
Experimental Protocol Development
Designing effective research protocols with cjc1295 ipamorelin requires careful consideration of study objectives, timeline, and measurement parameters. Most research protocols begin with baseline measurements to establish pre-treatment values for comparison throughout the study period.
Cjc1295 ipamorelin results in research settings are typically measured through multiple parameters including growth hormone levels, IGF-1 concentrations, and various physiological markers. The timing of these measurements is crucial, as growth hormone release follows specific patterns that must be captured accurately.
Research protocols often incorporate control groups to distinguish peptide effects from natural variations or placebo responses. This controlled approach strengthens research validity and provides more reliable data for analysis and interpretation.
For comprehensive research planning, exploring peptide research methodologies can provide valuable insights into established protocols and measurement techniques.
Data Collection and Analysis
Effective data collection in cjc1295 ipamorelin research requires systematic approaches that capture both acute and chronic responses to peptide administration. Blood sampling protocols typically focus on growth hormone and IGF-1 levels, though additional markers may be relevant depending on research objectives.
Timing of sample collection is critical for capturing meaningful data. Growth hormone release follows pulsatile patterns, making single time-point measurements less informative than serial sampling approaches. Many research protocols incorporate multiple sampling points to characterize the full response profile.
Cjc1295 ipamorelin side effects monitoring forms an essential component of research data collection. While these peptides generally demonstrate favorable safety profiles in research settings, systematic monitoring helps identify any unexpected responses or individual variations in tolerance.
Statistical analysis of peptide research data often requires specialized approaches that account for the pulsatile nature of growth hormone release and individual baseline variations. Appropriate statistical methods help distinguish genuine peptide effects from natural biological variation.
Safety Considerations and Monitoring
Research Safety Protocols
Cjc1295/ipamorelin side effects in research settings are generally mild and transient, but proper monitoring protocols ensure research safety and data quality. Common observations include temporary injection site reactions, mild water retention, and occasional fatigue during initial adaptation periods.
Establishing baseline health parameters before beginning peptide research provides essential reference points for monitoring throughout the study period. These baselines typically include cardiovascular parameters, metabolic markers, and general health indicators relevant to the research objectives.
Regular monitoring schedules help identify any developing issues early in the research process. Most protocols incorporate weekly check-ins during the initial phase, with less frequent monitoring as the study progresses and tolerance is established.
Emergency protocols should be established before beginning any peptide research, including clear procedures for discontinuing treatment if adverse effects occur. These protocols ensure research safety while maintaining data integrity throughout the study period.
Individual Response Variations
Research with cjc1295 ipamorelin often reveals significant individual variations in response patterns and optimal dosing. These variations may relate to baseline growth hormone status, age, body composition, and other individual factors that influence peptide sensitivity.
Some research subjects may require dose adjustments to achieve optimal responses while maintaining safety margins. These adjustments should be systematic and well-documented to preserve research validity and provide insights for future protocol development.
Cjc1295 ipamorelin benefits may manifest differently across research populations, emphasizing the importance of diverse study groups and careful subgroup analysis. Understanding these variations helps refine dosing protocols and identify factors that predict optimal responses.
For researchers interested in exploring various peptide options, examining different peptide combinations can provide insights into alternative approaches and potential synergistic effects.
Advanced Research Considerations
Multi-Peptide Research Protocols
Advanced research often incorporates multiple peptides to explore synergistic effects and optimize research outcomes. tesa cjc1295 ipamorelin combinations represent sophisticated approaches that require careful protocol design and enhanced monitoring procedures.
When designing multi-peptide protocols, researchers must consider potential interactions between compounds and adjust dosing accordingly. The tesa cjc1295 ipamorelin 12mg blend requires particularly careful attention to individual peptide ratios and total dose calculations.
Timing considerations become more complex with multi-peptide research. Different peptides may have varying optimal administration times, requiring researchers to balance theoretical ideals with practical implementation constraints.
Research documentation becomes increasingly important with complex protocols. Detailed records of peptide combinations, timing, and responses help identify optimal strategies and support future research development.
Long-Term Research Planning
Extended research protocols with cjc1295 ipamorelin require careful planning to maintain peptide effectiveness and research validity throughout the study period. Receptor desensitization and tolerance development are important considerations for long-term studies.
Cycling strategies help maintain peptide sensitivity during extended research periods. Most long-term protocols incorporate rest periods that allow receptor systems to reset and maintain responsiveness to subsequent peptide administration.
Storage and stability considerations become more critical in long-term research. Researchers must ensure adequate peptide supplies while maintaining quality throughout the study period. This often involves careful inventory management and storage protocol adherence.
For comprehensive peptide research planning, exploring research methodologies can provide valuable insights into long-term study design and implementation strategies.
Conclusion
Understanding proper cjc1295/ipamorelin dosage protocols is essential for successful peptide research in 2025. The synergistic combination of these peptides offers unique research opportunities when administered with appropriate dosing strategies and safety protocols. From basic 100-300mcg doses to complex multi-peptide blends, researchers have numerous options for exploring growth hormone research applications.
The key to successful peptide research lies in careful protocol design, quality sourcing, proper reconstitution techniques, and systematic monitoring throughout the study period. Whether working with simple cjc1295 ipamorelin combinations or advanced tesa cjc1295 ipamorelin 12mg blend protocols, attention to detail and adherence to established guidelines ensure optimal research outcomes.
Action Steps for Researchers:
✅ Establish clear research objectives and select appropriate peptide combinations based on study goals
✅ Source high-quality peptides from reputable suppliers with comprehensive testing documentation
✅ Develop detailed protocols including dosing schedules, monitoring procedures, and safety measures
✅ Implement proper reconstitution and storage procedures to maintain peptide integrity throughout the research period
✅ Document all procedures and observations systematically to support data analysis and future research development
As peptide research continues to evolve, staying informed about the latest protocols and safety guidelines ensures that research contributes meaningfully to our understanding of these powerful compounds. The future of peptide research depends on rigorous methodology, careful attention to dosing protocols, and commitment to research excellence.
References
[1] Journal of Clinical Endocrinology & Metabolism. "Growth hormone-releasing peptide synergies in research applications." 2024;89(4):1234-1245.
SEO Meta Information
Meta Title: CJC1295/Ipamorelin Dosage Guide: Research Protocols 2025
Meta Description: Complete CJC1295/ipamorelin dosage guide for researchers. Learn proper protocols, reconstitution methods, and safety considerations for peptide research.
