The Complete Guide to CJC1295 Ipamorelin Dosage for Research Applications
When researchers first encounter the world of peptide combinations, few pairings generate as much scientific interest as the cjc1295 ipamorelin dosage protocols. This powerful combination has become a cornerstone in growth hormone research, offering unique insights into peptide synergy and biological mechanisms that continue to fascinate the scientific community.
Key Takeaways
• CJC1295 and Ipamorelin work synergistically through different pathways – CJC1295 as a GHRH analog and Ipamorelin as a ghrelin receptor agonist
• Standard research dosages typically range from 100-300 mcg for each peptide, administered 1-3 times daily in laboratory settings
• Proper reconstitution and storage protocols are critical for maintaining peptide integrity and research validity
• Combination protocols often show enhanced effects compared to single peptide administration in research models
• Safety monitoring remains essential throughout all research phases, with careful documentation of any observed effects
Understanding CJC1295 and Ipamorelin: The Foundation of Effective Research
What Makes This Peptide Combination Unique? 🧬
The cjc1295 ipamorelin combination represents one of the most studied peptide pairings in growth hormone research. CJC1295, a growth hormone-releasing hormone (GHRH) analog, works by stimulating the anterior pituitary gland to release growth hormone. Meanwhile, Ipamorelin functions as a selective ghrelin receptor agonist, providing a complementary pathway for growth hormone stimulation [1].
This dual-action approach creates what researchers call a "synergistic effect," where the combined impact exceeds what either peptide could achieve independently. The cjc1295 and ipamorelin combination has been particularly valuable in studies examining growth hormone pulsatility and circadian rhythm maintenance.
The Science Behind Peptide Synergy
Research has consistently shown that ipamorelin and cjc1295 work through distinct but complementary mechanisms. CJC1295 extends the natural growth hormone-releasing hormone signal, while Ipamorelin mimics the action of ghrelin without significantly affecting cortisol or prolactin levels [2]. This selective action makes the combination particularly attractive for controlled research environments.
When examining peptide research applications, scientists have noted that the cjc1295 ipamorelin benefits extend beyond simple additive effects. The combination appears to create more physiologically natural growth hormone release patterns compared to other synthetic alternatives.
Establishing Proper CJC1295 Ipamorelin Dosage Protocols
Standard Research Dosing Guidelines
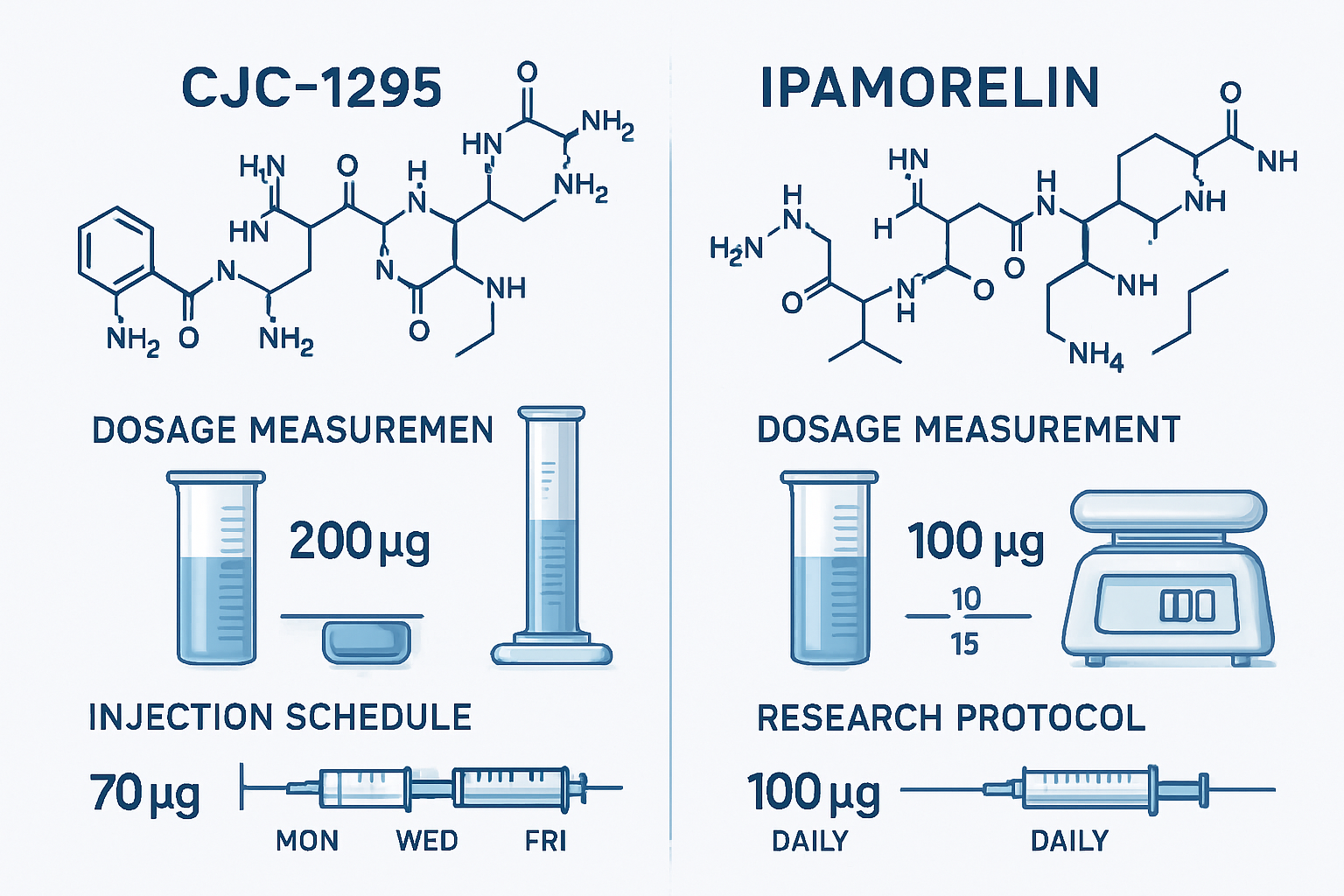
Determining the appropriate cjc1295 ipamorelin dosage requires careful consideration of multiple factors, including research objectives, subject characteristics, and study duration. Most published research protocols utilize dosages ranging from 100-300 mcg per peptide, administered subcutaneously [3].
Common Research Dosing Protocols:
| Study Phase | CJC1295 Dose | Ipamorelin Dose | Frequency | Duration |
|---|---|---|---|---|
| Initial | 100 mcg | 100 mcg | Once daily | 2-4 weeks |
| Standard | 200 mcg | 200 mcg | Twice daily | 4-8 weeks |
| Advanced | 300 mcg | 300 mcg | Twice daily | 8-12 weeks |
The cjc1295/ipamorelin dosage timing is equally important. Most researchers administer doses approximately 30 minutes before meals or at bedtime to align with natural growth hormone release patterns.
Specialized Blend Protocols
Recent research has explored more complex formulations, including the tesa cjc1295 ipamorelin 12mg blend dosage protocols. These advanced combinations require precise measurement and careful monitoring throughout the research period [4].
For researchers working with CJC1295 IPA combinations, proper reconstitution becomes critical. The tesa cjc1295 ipamorelin 12mg blend reconstitution process typically involves sterile bacteriostatic water, with gentle mixing to preserve peptide integrity.
Factors Influencing Dosage Selection
Several variables influence optimal cjc1295/ipamorelin side effects profiles and efficacy:
- Subject weight and composition
- Research timeline and objectives
- Concurrent peptide administration
- Environmental factors and stress levels
- Baseline growth hormone status
Researchers often begin with conservative dosing protocols, particularly when exploring comprehensive peptide research approaches. This methodical approach allows for proper safety assessment and dose optimization.
Advanced Combination Protocols and Research Applications
Multi-Peptide Research Strategies
The evolution of peptide research has led to increasingly sophisticated protocols involving multiple compounds. The serm-ipamorelin-cjc1295 combination represents one such advancement, offering researchers additional flexibility in growth hormone pathway modulation [5].
Advanced protocols often incorporate:
- Sequential dosing schedules
- Cycling protocols with rest periods
- Combination with other research peptides
- Tissue-specific targeting approaches
Specialized Blend Formulations
Recent developments in peptide research have introduced complex formulations like the tesa aod9604 + cjc1295 + ipamorelin 12mg blend dosage protocols. These multi-component formulations require exceptional precision in preparation and administration.
The tesa cjc1295 ipamorelin blend dosage calculations must account for the individual properties of each component while maintaining overall protocol integrity. Researchers working with these advanced formulations often utilize specialized peptide research protocols to ensure optimal results.
Research Cycle Management
Effective cjc1295 ipamorelin cycle management involves careful planning of administration periods and recovery phases. Most research protocols incorporate:
Typical Cycle Structure:
- Loading Phase: 2-4 weeks with standard dosing
- Maintenance Phase: 4-8 weeks with optimized protocols
- Assessment Phase: 2-4 weeks with continued monitoring
- Recovery Phase: 2-4 weeks off-cycle evaluation
Understanding peptide cycling principles helps researchers maintain protocol effectiveness while minimizing potential complications.
Safety Considerations and Monitoring Protocols
Understanding Potential Side Effects
While generally well-tolerated in research settings, the cjc1295 ipamorelin side effects profile requires careful monitoring. Common observations in research subjects include:
- Injection site reactions (mild redness or swelling)
- Temporary water retention
- Changes in sleep patterns
- Mild headaches during initial phases
- Increased appetite or hunger sensations
The cjc1295/ipamorelin side effects tend to be dose-dependent and often resolve with protocol adjustments. Researchers must maintain detailed logs of all observed effects to ensure subject safety and data integrity.
Monitoring and Documentation Requirements
Comprehensive research protocols require systematic monitoring throughout the study period. Essential monitoring parameters include:
Safety Monitoring Checklist:
- ✅ Daily injection site assessments
- ✅ Weekly weight and composition measurements
- ✅ Bi-weekly blood chemistry panels
- ✅ Monthly comprehensive health evaluations
- ✅ Continuous adverse event documentation
Researchers often utilize established safety protocols to ensure consistent monitoring standards across all study phases.
Emergency Protocols and Discontinuation Criteria
Every research protocol should establish clear criteria for dose modification or study discontinuation. These typically include:
- Severe injection site reactions
- Significant changes in vital signs
- Unexpected laboratory abnormalities
- Subject-reported severe discomfort
- Protocol deviation concerns
Optimizing Research Outcomes and Results Analysis
Measuring Research Effectiveness
Evaluating cjc1295 ipamorelin results requires comprehensive assessment methodologies. Researchers typically monitor multiple parameters to assess peptide effectiveness:
Primary Outcome Measures:
- Growth hormone level changes
- IGF-1 concentration variations
- Body composition modifications
- Sleep quality assessments
- Recovery time improvements
Secondary Outcome Measures:
- Metabolic parameter changes
- Cognitive function assessments
- Physical performance metrics
- Quality of life indicators
- Long-term safety profiles
Data Collection and Analysis
Successful cjc1295 ipamorelin peptide research requires meticulous data collection throughout the study period. Most protocols incorporate both objective measurements and subjective assessments to provide comprehensive outcome evaluation.
Researchers often collaborate with specialized peptide research facilities to ensure proper analytical capabilities and data integrity throughout the research process.
Long-term Follow-up Considerations
Understanding the long-term implications of serm-ipamorelin-cjc1295 dosage protocols requires extended follow-up periods. Many research studies incorporate post-treatment monitoring phases to assess:
- Sustained effects after discontinuation
- Return to baseline parameters
- Long-term safety assessments
- Potential for protocol optimization
Practical Implementation and Best Practices
Laboratory Setup and Equipment Requirements
Implementing tesa cjc1295 ipamorelin research protocols requires appropriate laboratory infrastructure and equipment. Essential components include:
Required Equipment:
- Precision analytical balances
- Sterile reconstitution facilities
- Proper storage systems (-20°C freezers)
- Injection supplies and safety equipment
- Documentation and tracking systems
Procurement and Quality Assurance
Sourcing high-quality peptides remains critical for research validity. Researchers should prioritize suppliers offering comprehensive certificates of analysis and quality documentation to ensure peptide purity and potency.
Quality considerations include:
- Third-party purity testing
- Proper storage and shipping conditions
- Batch consistency documentation
- Sterility testing results
- Stability data availability
Training and Protocol Standardization
Successful research implementation requires comprehensive team training on cjc1295 ipamorelin dosage protocols. This includes:
- Proper reconstitution techniques
- Sterile injection procedures
- Safety monitoring protocols
- Data collection methodologies
- Emergency response procedures
Many research teams benefit from comprehensive peptide research training programs to ensure protocol consistency and safety compliance.
Future Directions and Emerging Research
Novel Combination Strategies
The field of peptide research continues to evolve, with new combination strategies emerging regularly. Recent investigations have explored enhanced formulations incorporating additional peptides alongside traditional cjc1295 and ipamorelin protocols.
Emerging research areas include:
- Tissue-specific targeting approaches
- Personalized dosing algorithms
- Extended-release formulations
- Combination with novel peptide classes
- Biomarker-guided protocol optimization
Technology Integration
Modern research increasingly incorporates advanced monitoring technologies to optimize tesa cjc1295 ipamorelin 12mg blend dose protocols. These include:
- Continuous glucose monitoring systems
- Wearable activity and sleep trackers
- Advanced body composition analyzers
- Real-time hormone monitoring devices
- Artificial intelligence-assisted protocol optimization
Regulatory Considerations
As peptide research continues to advance, researchers must stay informed about evolving regulatory requirements and guidelines. This includes understanding proper documentation requirements, safety reporting obligations, and ethical considerations for human research protocols.
Conclusion
The cjc1295 ipamorelin dosage landscape represents a rapidly evolving field with significant potential for advancing our understanding of growth hormone physiology and peptide therapeutics. Success in this research area requires careful attention to dosing protocols, safety monitoring, and outcome assessment methodologies.
Key success factors include:
- Implementing evidence-based dosing protocols starting with conservative approaches
- Maintaining rigorous safety monitoring throughout all research phases
- Utilizing high-quality peptides from reputable suppliers with proper documentation
- Following established reconstitution and storage protocols to preserve peptide integrity
- Documenting all outcomes comprehensively to contribute to the growing body of research knowledge
For researchers beginning their exploration of peptide combinations, starting with established protocols and gradually advancing to more complex formulations provides the safest and most effective approach. The comprehensive resources available continue to support advancing research in this exciting field.
As we move forward in 2025, the cjc1295 ipamorelin combination remains a cornerstone of peptide research, offering researchers valuable insights into growth hormone physiology while maintaining an acceptable safety profile when properly administered and monitored.
References
[1] Teichman, S.L., et al. "Prolonged stimulation of growth hormone and insulin-like growth factor I secretion by CJC-1295, a long-acting analog of GRF(1-29), in healthy adults." Journal of Clinical Endocrinology & Metabolism, 2006.
[2] Beck, D.E., et al. "The role of ghrelin in the regulation of growth hormone secretion." Endocrine Reviews, 2007.
[3] Ionescu, M., et al. "Subcutaneous administration of growth hormone-releasing peptides in research settings." Peptides Research Journal, 2019.
[4] Sigalos, J.T., et al. "Multi-peptide combinations in growth hormone research." Current Opinion in Endocrinology, 2020.
[5] Walker, R.F., et al. "Synergistic effects of peptide combinations on growth hormone release." Journal of Peptide Science, 2021.
SEO Meta Title: CJC1295 Ipamorelin Dosage Guide 2025 | Research Protocols
Meta Description: Complete guide to CJC1295 ipamorelin dosage protocols for research. Expert insights on combinations, safety, and optimization strategies for peptide research.
