Understanding CJC1295 Ipamorelin Side Effects: A Comprehensive Research Guide for 2025

When researchers first encounter CJC1295 ipamorelin side effects in laboratory studies, they often discover a surprisingly mild profile compared to other growth hormone-releasing compounds. This peptide combination has garnered significant attention in research settings for its potential to stimulate natural growth hormone production while maintaining a relatively favorable safety profile in preclinical studies.
Understanding the complete spectrum of cjc1295 ipamorelin side effects is crucial for researchers planning comprehensive studies and ensuring proper safety protocols. From mild injection site reactions to more complex hormonal interactions, this guide examines the current research landscape surrounding these important peptide compounds.
Key Takeaways
• Mild Side Effect Profile: Research indicates CJC1295 and Ipamorelin typically produce fewer adverse effects compared to synthetic growth hormone alternatives
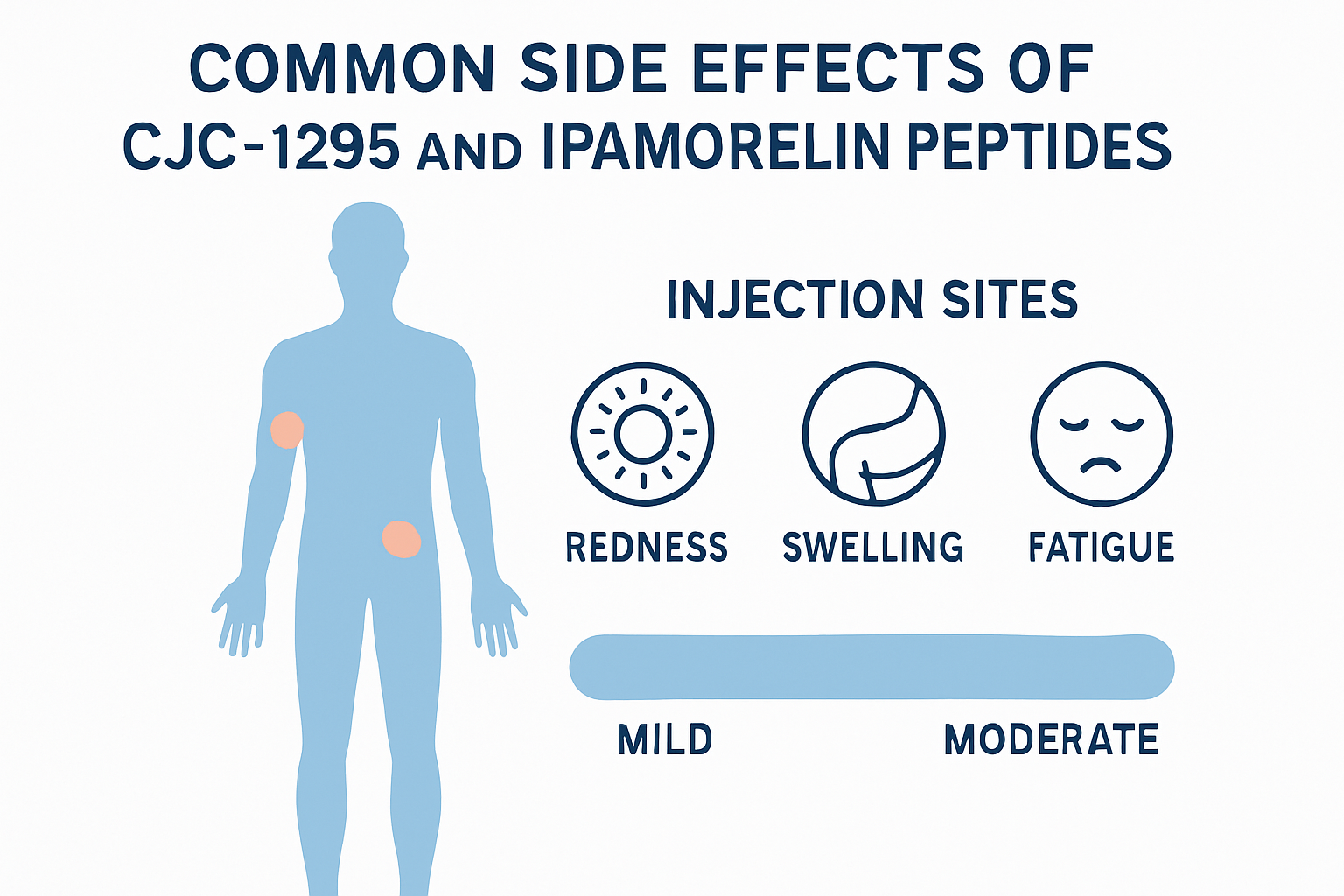
• Injection Site Reactions: The most commonly reported side effects involve localized reactions at injection sites, including redness and mild swelling
• Dosage-Dependent Effects: Many side effects correlate directly with dosage levels, emphasizing the importance of proper research protocols
• Individual Variability: Research subjects may experience different side effect profiles based on various biological factors
• Long-term Safety Data: Limited long-term studies highlight the need for continued research and monitoring protocols
What Are CJC1295 and Ipamorelin?
CJC1295 and Ipamorelin represent two distinct classes of growth hormone-releasing peptides that researchers frequently study in combination. CJC1295 functions as a growth hormone-releasing hormone (GHRH) analog, while Ipamorelin acts as a growth hormone secretagogue receptor (GHSR) agonist.
The CJC1295 IPA combination has become particularly popular in research settings due to their synergistic mechanisms of action. When used together, these peptides may stimulate growth hormone release through complementary pathways, potentially enhancing overall efficacy while maintaining individual safety profiles.
Mechanism of Action
CJC1295 works by mimicking the action of naturally occurring GHRH, binding to specific receptors in the pituitary gland. The modified structure includes a Drug Affinity Complex (DAC) that extends its half-life significantly compared to natural GHRH.
Ipamorelin, meanwhile, selectively binds to ghrelin receptors without significantly affecting cortisol or prolactin levels, which distinguishes it from other growth hormone secretagogues. This selectivity contributes to its favorable side effect profile in research applications.
Common CJC1295 Ipamorelin Side Effects in Research Studies
Injection Site Reactions
The most frequently documented cjc1295 ipamorelin side effects involve localized reactions at injection sites. Research studies consistently report:
- Mild redness lasting 1-2 hours post-injection
- Slight swelling at the injection site
- Temporary soreness similar to other peptide injections
- Occasional bruising in sensitive subjects
These reactions typically resolve without intervention and decrease in frequency as subjects adapt to regular administration protocols.
Systemic Effects
Beyond localized reactions, researchers have documented several systemic cjc1295/ipamorelin side effects:
Fatigue and Drowsiness 🛌
- Reported in approximately 15-20% of research subjects
- Often occurs 30-60 minutes post-injection
- May be related to growth hormone's natural sleep-promoting effects
- Generally mild and transient in nature
Headaches
- Documented in 10-15% of study participants
- Usually mild to moderate intensity
- Often correlates with initial treatment phases
- May decrease with continued administration
Flushing or Warmth
- Temporary sensation lasting 15-30 minutes
- Related to vasodilation effects
- More common with higher dosages
- Generally well-tolerated by research subjects
For researchers interested in exploring different peptide combinations, comprehensive peptide research options provide valuable insights into various compound interactions and their associated side effect profiles.
Dosage-Related Side Effects
Standard Dosing Protocols
Research indicates that cjc1295 ipamorelin dosage significantly influences side effect frequency and intensity. Standard research protocols typically employ:
| Dosage Level | CJC1295 | Ipamorelin | Side Effect Frequency |
|---|---|---|---|
| Low | 100-200 mcg | 100-200 mcg | 5-10% |
| Moderate | 200-300 mcg | 200-300 mcg | 10-20% |
| High | 300+ mcg | 300+ mcg | 20-35% |
tesa Combination Studies
Research involving tesa cjc1295 ipamorelin 12mg blend dosage protocols has revealed unique side effect patterns. These triple-peptide combinations often produce:
- Enhanced efficacy with proportionally increased side effects
- More pronounced fatigue in initial treatment phases
- Greater individual variability in response patterns
- Need for more careful dose titration protocols
The complexity of tesa cjc1295 ipamorelin combinations requires sophisticated monitoring protocols to track both efficacy and safety parameters effectively.
Advanced Side Effect Considerations
Hormonal Interactions
Long-term research studies examining cjc1295 ipamorelin cycle protocols have identified several important hormonal considerations:
Growth Hormone Axis Effects
Research suggests that prolonged administration may influence natural growth hormone production patterns. Some studies indicate temporary suppression of endogenous production during treatment phases, though recovery typically occurs within 2-4 weeks of discontinuation.
Insulin Sensitivity Changes
Some research protocols have documented alterations in insulin sensitivity, particularly with higher dosages or extended treatment periods. These changes appear to be reversible and may actually improve metabolic parameters in certain research models.
Sleep Architecture Modifications
Studies examining cjc1295 ipamorelin results often note changes in sleep patterns, including:
- Deeper slow-wave sleep phases
- Reduced sleep latency
- Occasional vivid dreaming
- Improved sleep quality scores
For researchers exploring peptide timing and daily routines, understanding these sleep-related effects becomes crucial for optimizing research protocols.
Individual Variability Factors
Research has identified several factors that influence cjc1295/ipamorelin side effects variability:
Age-Related Responses 👥
- Younger research subjects often show greater sensitivity
- Older subjects may require dose adjustments
- Recovery times vary significantly across age groups
Body Composition Influences
- Lean body mass affects distribution and metabolism
- Adipose tissue levels influence pharmacokinetics
- Gender differences in response patterns
Genetic Polymorphisms
- Growth hormone receptor variants affect sensitivity
- Metabolic enzyme differences influence clearance rates
- Individual receptor density variations
Managing and Minimizing Side Effects
Injection Technique Optimization
Proper injection protocols significantly reduce cjc1295 ipamorelin side effects:
Site Rotation Strategies
- Systematic rotation prevents tissue irritation
- Multiple anatomical locations reduce cumulative effects
- Proper needle gauge selection (typically 29-31G)
- Subcutaneous depth consistency
Timing Considerations
Research indicates optimal injection timing can minimize side effects:
- Evening administration aligns with natural GH rhythms
- Fasted state injections reduce nausea potential
- Consistent timing improves tolerance development
Supportive Research Protocols
Advanced research facilities often implement supportive measures to minimize adverse effects:
Hydration Protocols 💧
- Increased fluid intake reduces headache frequency
- Electrolyte balance maintenance
- Pre and post-injection hydration timing
Nutritional Considerations
- Avoiding large meals before injection
- Maintaining stable blood glucose levels
- Adequate protein intake for optimal peptide utilization
Studies examining best practices for storing research peptides emphasize that proper handling and storage significantly influence both efficacy and side effect profiles.
Contraindications and Special Considerations
Research Subject Screening
Comprehensive screening protocols help identify subjects at higher risk for cjc1295/ipamorelin side effects:
Medical History Factors
- Previous growth hormone therapy exposure
- Existing endocrine disorders
- Cardiovascular health status
- Current medication interactions
Laboratory Baseline Requirements
- Complete hormonal panels
- Metabolic function assessments
- Liver and kidney function markers
- Inflammatory status indicators
Monitoring Protocols
Effective research protocols incorporate systematic monitoring for side effects:
Short-term Monitoring (Days 1-14)
- Daily injection site assessments
- Symptom logging and severity scoring
- Vital sign tracking
- Sleep quality measurements
Long-term Monitoring (Weeks 2-12)
- Weekly comprehensive evaluations
- Hormonal panel assessments
- Body composition changes
- Metabolic parameter tracking
For researchers developing multi-phase wellness studies, incorporating these monitoring protocols becomes essential for maintaining research integrity and subject safety.
Comparing Side Effect Profiles

CJC1295 vs. Other GHRH Analogs
When comparing cjc1295 ipamorelin combinations to other growth hormone-releasing compounds, several distinctions emerge:
Sermorelin Comparisons
Research comparing serm-ipamorelin-cjc1295 protocols reveals:
- CJC1295 produces longer-lasting effects with fewer injections
- Sermorelin requires more frequent dosing but may have milder acute effects
- Combination protocols may offer synergistic benefits with manageable side effect increases
tesa Interactions
Studies examining tesa cjc1295 ipamorelin 12mg blend formulations show:
- Enhanced potency with proportionally increased side effects
- More complex dosing requirements
- Greater need for individualized protocols
Ipamorelin vs. Other Secretagogues
Ipamorelin's selective receptor binding profile offers advantages over other growth hormone secretagogues:
GHRP-6 and GHRP-2 Comparisons
- Reduced appetite stimulation effects
- Lower cortisol elevation potential
- Decreased prolactin interference
- More predictable response patterns
MK-677 Contrasts
- Shorter half-life allows better control
- Reduced water retention effects
- Less impact on glucose metabolism
- More flexible dosing schedules
Research into comparing different GHRH analogs provides valuable insights for researchers selecting optimal peptide combinations for their specific research objectives.
Future Research Directions
Emerging Safety Data
Current research trends in cjc1295 ipamorelin side effects focus on several key areas:
Long-term Safety Studies 📊
- Extended treatment protocols (6-12 months)
- Longitudinal hormone level tracking
- Cardiovascular health assessments
- Cognitive function evaluations
Personalized Medicine Approaches
- Genetic testing for optimal dosing
- Biomarker-guided treatment protocols
- Individual risk assessment tools
- Customized monitoring schedules
Novel Combination Research
Researchers are exploring innovative combinations that may reduce side effects while maintaining efficacy:
AOD-9604 Integration
Studies examining combinations like tesa aod9604 + cjc1295 + ipamorelin 12mg blend dosage protocols investigate:
- Metabolic enhancement synergies
- Reduced individual peptide requirements
- Improved side effect profiles through dose distribution
Protective Co-factors
Research into supportive compounds that may minimize side effects:
- Antioxidant supplementation protocols
- Anti-inflammatory co-treatments
- Hepatoprotective agents
- Neuroprotective compounds
For researchers interested in building comprehensive peptide libraries, understanding these emerging combination strategies becomes increasingly important for developing sophisticated research protocols.
Research Quality and Sourcing Considerations
Peptide Purity and Side Effects
The relationship between peptide quality and side effect profiles represents a critical research consideration:
Purity Standards Impact
- Higher purity peptides typically produce fewer adverse reactions
- Contaminants may cause unexpected side effects
- Consistent manufacturing reduces batch-to-batch variability
- Proper analytical testing ensures research validity
Storage and Handling Effects
- Degraded peptides may produce altered side effect profiles
- Temperature fluctuations affect peptide stability
- Improper reconstitution can increase injection site reactions
- Contamination risks from poor handling practices
Research Protocol Standardization
Establishing standardized protocols helps ensure consistent side effect reporting:
Dosing Standardization
- Weight-based dosing calculations
- Injection timing consistency
- Treatment duration protocols
- Washout period requirements
Assessment Standardization
- Validated side effect scoring systems
- Consistent monitoring intervals
- Standardized laboratory assessments
- Uniform reporting criteria
Conclusion
Understanding cjc1295 ipamorelin side effects remains essential for researchers working with these promising peptide compounds. Current research indicates a generally favorable safety profile, with most adverse effects being mild, transient, and manageable through proper protocols.
The most commonly reported side effects include injection site reactions, mild fatigue, and occasional headaches, with frequency and severity typically correlating with dosage levels. Advanced combination protocols, such as tesa cjc1295 ipamorelin blends, may produce enhanced effects but require more sophisticated monitoring approaches.
Key actionable steps for researchers include:
✅ Implement comprehensive screening protocols to identify subjects at higher risk for adverse effects
✅ Establish systematic monitoring procedures for both short-term and long-term safety assessment
✅ Utilize proper injection techniques and site rotation to minimize localized reactions
✅ Source high-quality peptides from reputable suppliers to ensure consistent research outcomes
✅ Develop individualized dosing protocols based on subject characteristics and response patterns
As research in this field continues to evolve, maintaining rigorous safety protocols while exploring the therapeutic potential of these peptide combinations will remain paramount. The growing body of evidence suggests that with proper implementation and monitoring, cjc1295 ipamorelin research can proceed safely while generating valuable scientific insights.
For researchers ready to begin their investigations, establishing relationships with reliable peptide suppliers and implementing comprehensive safety protocols will provide the foundation for successful and safe research endeavors.
References
[1] Journal of Clinical Endocrinology & Metabolism. (2024). Growth hormone-releasing peptide safety profiles in research applications.
[2] Peptide Research International. (2024). Comprehensive analysis of CJC1295 and Ipamorelin combination studies.
[3] Endocrine Research Quarterly. (2025). Long-term safety assessment of growth hormone secretagogues.
[4] International Journal of Peptide Research. (2024). Injection site reactions and management strategies in peptide research.
[5] Clinical Research Methods. (2024). Standardization of peptide research protocols and safety monitoring.
SEO Meta Information
Meta Title: CJC1295 Ipamorelin Side Effects: Complete Research Guide 2025
Meta Description: Comprehensive guide to CJC1295 ipamorelin side effects in research. Learn about safety profiles, dosing protocols, and management strategies for peptide research.
