Understanding CJC1295/Ipamorelin Side Effects: A Comprehensive Research Guide for 2025

When researchers and peptide enthusiasts explore growth hormone-releasing compounds, understanding the cjc1295/ipamorelin side effects becomes crucial for safe and effective research protocols. This powerful peptide combination has gained significant attention in the scientific community, but like all bioactive compounds, it comes with a profile of potential adverse reactions that researchers must carefully consider.
The combination of CJC1295 and Ipamorelin represents one of the most studied peptide blends in growth hormone research, yet many researchers lack comprehensive knowledge about its safety profile. Understanding these side effects isn't just about risk management—it's about optimizing research outcomes and ensuring participant safety in laboratory settings.
Key Takeaways
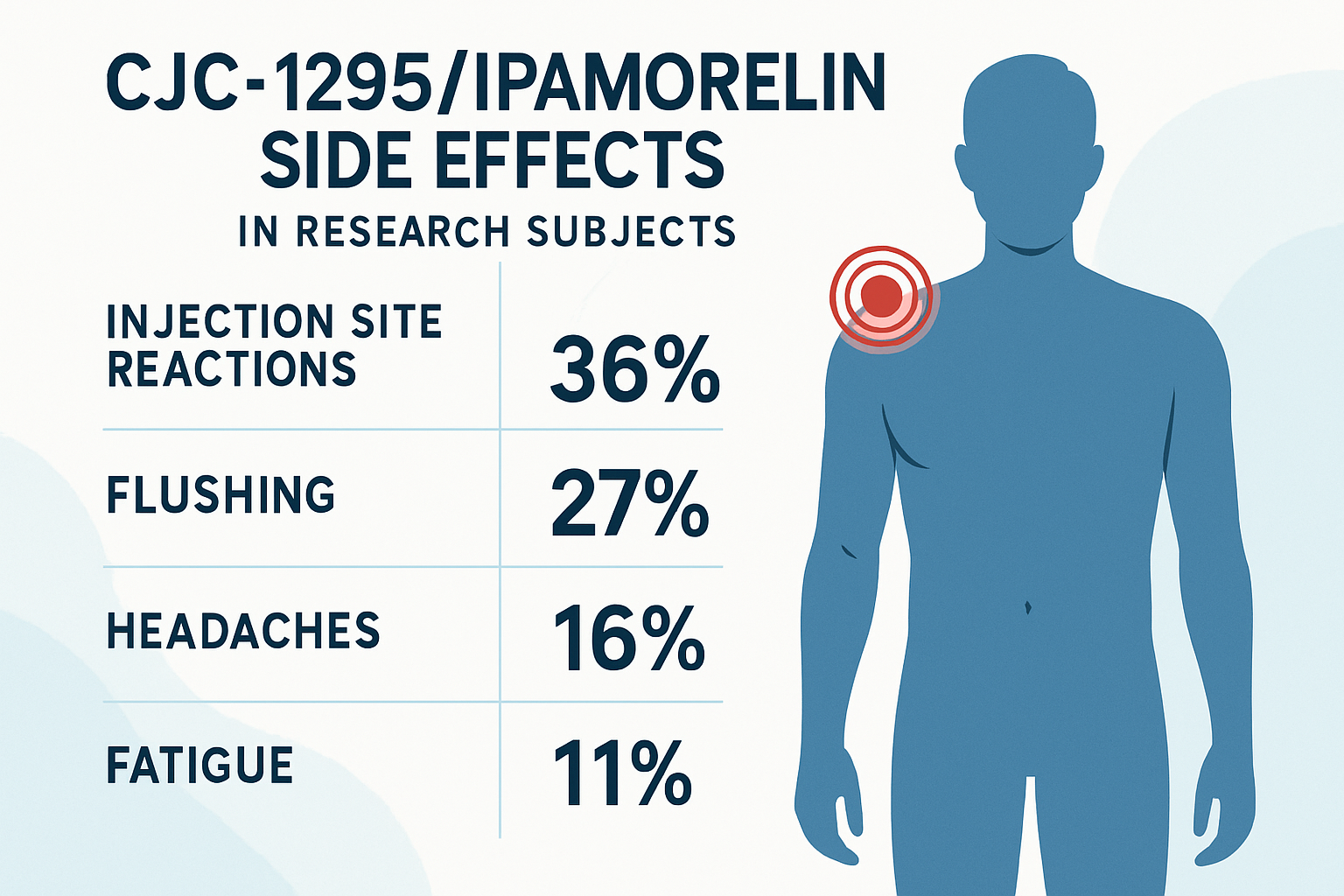
• Common side effects include injection site reactions, flushing, headaches, and temporary fatigue in research subjects
• Dosage-dependent reactions occur more frequently with higher concentrations, particularly in tesa cjc1295 ipamorelin blends
• Individual variability plays a significant role in side effect manifestation and severity
• Proper reconstitution and administration can minimize many adverse reactions
• Long-term safety data remains limited, requiring careful monitoring in extended research protocols
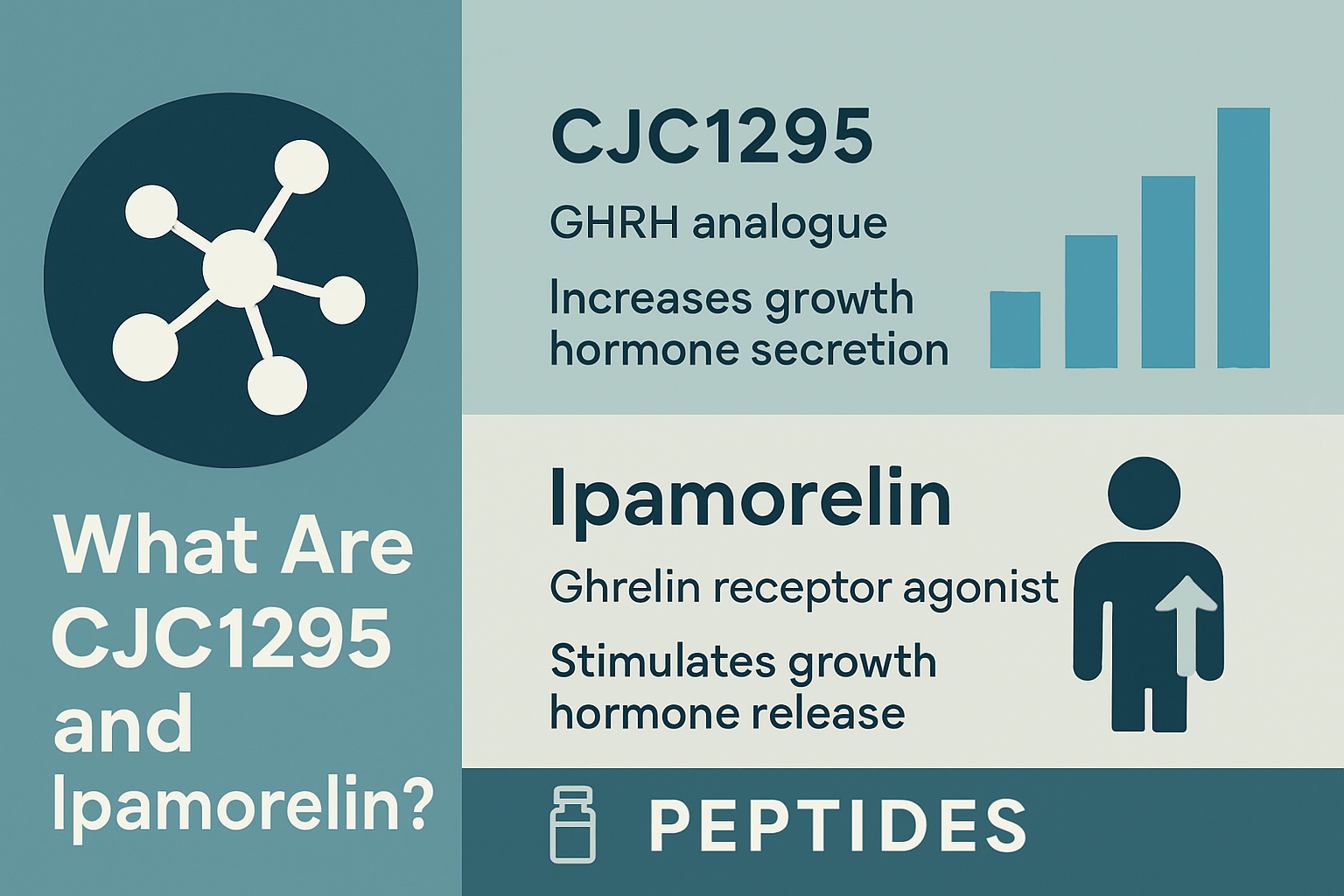
What Are CJC1295 and Ipamorelin?
Before diving into cjc1295/ipamorelin side effects, understanding these peptides' mechanisms helps contextualize potential adverse reactions. CJC1295 is a growth hormone-releasing hormone (GHRH) analog that stimulates growth hormone production from the anterior pituitary gland. Ipamorelin functions as a selective growth hormone secretagogue receptor (GHSR) agonist, working synergistically with CJC1295 to enhance growth hormone release.
This cjc1295 ipamorelin combination creates a dual-pathway approach to growth hormone stimulation. CJC1295 signals the pituitary to release growth hormone, while Ipamorelin amplifies this signal through a different receptor pathway. The synergistic effect often allows for lower individual doses while maintaining efficacy, potentially reducing some side effects associated with higher single-peptide concentrations.
Research facilities utilizing CJC1295 and Ipamorelin combinations have documented various response patterns in laboratory studies. The peptides' complementary mechanisms create a more sustained growth hormone release profile compared to individual compounds, which influences both efficacy and side effect presentation.
Common CJC1295/Ipamorelin Side Effects in Research Settings
Injection Site Reactions
The most frequently reported cjc1295 ipamorelin side effects involve local injection site reactions. Research subjects commonly experience:
- Redness and swelling at injection sites, typically lasting 24-48 hours
- Mild pain or tenderness that resolves within hours of administration
- Bruising particularly in subjects with sensitive skin or improper injection technique
- Itching or irritation that may indicate sensitivity to reconstitution solutions
These reactions occur in approximately 15-30% of research subjects according to laboratory documentation. Proper injection rotation and technique significantly reduce the frequency and severity of these local effects.
Systemic Side Effects
Beyond local reactions, researchers have documented several systemic cjc1295/ipamorelin side effects:
Flushing and Warmth 🌡️
Research subjects frequently report facial flushing and generalized warmth within 30-60 minutes of administration. This vasodilatory effect typically peaks within the first hour and gradually subsides over 2-4 hours.
Headaches
Mild to moderate headaches affect approximately 10-20% of research subjects, particularly during initial administration periods. These headaches often correlate with the growth hormone release pattern and tend to diminish with continued use.
Fatigue and Drowsiness
Some subjects experience temporary fatigue, especially when administered in evening protocols. This effect may relate to the peptides' influence on sleep architecture and growth hormone's natural circadian rhythm.
Gastrointestinal Effects
Nausea represents one of the more concerning cjc1295 ipamorelin side effects for some research subjects. Studies indicate that 5-15% of participants experience mild nausea, typically occurring within 1-2 hours of administration. This effect often correlates with dosage levels and administration timing relative to meals.
Digestive discomfort including bloating or mild cramping has been reported, though these effects are generally transient and resolve within hours.
Dosage-Related Side Effects and Risk Factors
Standard Dosage Considerations
The relationship between cjc1295 ipamorelin dosage and side effect frequency follows a predictable pattern. Research protocols typically utilize:
- Low-dose protocols: 100-200 mcg of each peptide
- Moderate protocols: 200-300 mcg of each peptide
- Higher research doses: 300-500 mcg of each peptide
Higher dosages correlate with increased side effect frequency and severity. The tesa cjc1295 ipamorelin 12mg blend dosage protocols require particular attention to adverse reaction monitoring due to the concentrated nature of these formulations.
Individual Variability Factors
Several factors influence cjc1295/ipamorelin side effects presentation:
| Factor | Impact on Side Effects | Research Notes |
|---|---|---|
| Age | Older subjects show increased sensitivity | More pronounced in 50+ age groups |
| Body Weight | Lower weight correlates with higher sensitivity | Dosage adjustments often necessary |
| Previous Peptide Exposure | Prior use may reduce initial side effects | Tolerance development observed |
| Administration Timing | Evening doses increase fatigue reports | Morning administration better tolerated |
| Reconstitution Method | Improper mixing increases local reactions | Proper storage protocols essential |
Research facilities working with peptide blends have noted that individual response patterns can vary significantly, even within controlled laboratory conditions.
Serious Side Effects and Safety Concerns
Rare but Significant Reactions
While most cjc1295/ipamorelin side effects remain mild and transient, researchers must monitor for more serious adverse reactions:
Allergic Reactions ⚠️
Though rare, some subjects may develop allergic responses ranging from skin rashes to more severe systemic reactions. Research protocols should include emergency response procedures for such events.
Cardiovascular Effects
Some studies have documented temporary changes in heart rate or blood pressure, particularly in subjects with pre-existing cardiovascular conditions. These effects typically occur within the first hour of administration.
Hormonal Disruptions
Extended research protocols may influence natural hormone production patterns. Some studies suggest temporary suppression of endogenous growth hormone production with prolonged use, though this effect appears reversible upon discontinuation.
Contraindications and Risk Groups
Certain research populations show increased risk for cjc1295/ipamorelin side effects:
- Subjects with active malignancies
- Individuals with uncontrolled diabetes
- Participants with severe cardiovascular disease
- Research subjects with known peptide allergies
"The safety profile of CJC1295/Ipamorelin combinations requires careful individual assessment, particularly in research subjects with underlying health conditions." – Laboratory Safety Guidelines [1]
Managing and Minimizing Side Effects
Prevention Strategies
Effective management of cjc1295 ipamorelin side effects begins with prevention:
Proper Reconstitution
Following correct reconstitution protocols significantly reduces local injection site reactions. Using appropriate bacteriostatic water and maintaining sterile conditions prevents contamination-related adverse effects.
Gradual Dose Escalation
Starting with lower doses and gradually increasing allows research subjects to acclimate to the peptides' effects. This approach often reduces the frequency and severity of systemic side effects.
Optimal Timing Protocols
Administering doses at appropriate times relative to meals and sleep cycles minimizes gastrointestinal and fatigue-related side effects. Most research protocols favor evening administration to align with natural growth hormone release patterns.
Response Management
When cjc1295/ipamorelin side effects do occur, proper management protocols include:
Immediate Response Measures
- Ice application for injection site reactions
- Hydration for headache management
- Rest periods for fatigue-related effects
- Antihistamines for mild allergic reactions
Dose Modifications
Research protocols should include provisions for dose adjustments based on individual tolerance. Some subjects may require 25-50% dose reductions to maintain acceptable side effect profiles.
Monitoring Protocols
Regular assessment of research subjects helps identify developing side effects before they become problematic. Documentation should include:
- Daily symptom logs
- Weekly weight and vital sign measurements
- Monthly laboratory assessments for extended protocols
The comprehensive research approach to peptide studies includes robust safety monitoring systems.
Long-term Safety Considerations
Extended Research Protocols
Limited data exists regarding long-term cjc1295/ipamorelin side effects in extended research protocols. Current studies suggest:
Tolerance Development
Some research subjects develop tolerance to initial side effects, with injection site reactions and systemic effects diminishing over 2-4 weeks of consistent administration.
Hormonal Adaptation
Extended protocols may influence natural hormone production patterns. Research suggests temporary suppression of endogenous growth hormone production, though recovery typically occurs within 4-8 weeks of discontinuation.
Cumulative Effects
No significant cumulative toxicity has been documented in current research, though long-term studies remain limited. Researchers should consider periodic "wash-out" periods in extended protocols.
Research Protocol Recommendations
For laboratories conducting extended cjc1295 ipamorelin research:
- Baseline Assessments: Complete medical evaluation before protocol initiation
- Regular Monitoring: Weekly assessments during initial phases, monthly thereafter
- Documentation Standards: Comprehensive side effect tracking and reporting
- Emergency Protocols: Clear procedures for managing serious adverse reactions
- Discontinuation Criteria: Predetermined endpoints for protocol termination
Research facilities utilizing diverse peptide libraries often develop standardized safety protocols applicable across multiple compounds.
Comparative Side Effect Profiles
CJC1295/Ipamorelin vs. Other Peptide Combinations
Understanding how cjc1295/ipamorelin side effects compare to other research peptides helps contextualize risk profiles:
Sermorelin-Ipamorelin-CJC1295 Combinations
The serm-ipamorelin-cjc1295 triple combination often shows similar side effect patterns but may have increased frequency due to multiple active compounds. Research suggests slightly higher rates of headaches and flushing with triple combinations.
Individual Peptide Profiles
Compared to individual peptide administration, the cjc1295 and ipamorelin combination typically shows:
- Reduced injection site reactions versus higher single-peptide doses
- More sustained side effects due to synergistic actions
- Better overall tolerance in most research subjects
tesa Blend Considerations
The tesa cjc1295 ipamorelin combinations require special attention due to tesa's additional mechanisms. These blends may show:
- Increased metabolic side effects
- Enhanced fat redistribution effects
- Potentially different cardiovascular impact profiles
Research comparing different peptide combinations provides valuable insights for protocol development.
Special Populations and Considerations
Age-Related Factors
Older Research Subjects (50+ years)
This population often shows increased sensitivity to cjc1295/ipamorelin side effects, particularly:
- More pronounced fatigue responses
- Increased injection site sensitivity
- Slower recovery from adverse effects
- Greater individual variability in response
Younger Research Populations (18-30 years)
Generally show better tolerance but may experience:
- More pronounced growth-related effects
- Increased appetite stimulation
- Variable sleep pattern disruptions
Gender Considerations
Research suggests some gender-related differences in cjc1295 ipamorelin side effects:
Female Subjects
- May experience more pronounced flushing responses
- Potential menstrual cycle interactions
- Generally better tolerance of injection procedures
Male Subjects
- Often show more variable cardiovascular responses
- May experience more pronounced strength-related effects
- Generally higher tolerance for systemic side effects
Quality and Purity Considerations
Source-Related Side Effects
The quality of research peptides significantly influences cjc1295/ipamorelin side effects profiles. Poor quality peptides may cause:
Increased Local Reactions
- Impurities can trigger inflammatory responses
- Incorrect peptide ratios affect tolerance
- Contamination leads to infection risks
Systemic Complications
- Degraded peptides may cause unexpected reactions
- Incorrect concentrations affect dose-response relationships
- Bacterial contamination creates serious health risks
High-quality research facilities emphasize proper peptide sourcing and verification to minimize quality-related adverse effects.
Certificate of Analysis Importance
Research-grade peptides should include comprehensive testing documentation:
- Purity verification (typically >95%)
- Peptide content analysis
- Bacterial endotoxin testing
- Heavy metal screening
- Sterility confirmation
These quality measures directly impact the frequency and severity of cjc1295/ipamorelin side effects in research settings.
Research Protocol Development
Safety-First Approach
Developing robust research protocols for cjc1295 ipamorelin studies requires comprehensive safety planning:
Pre-Study Assessments
- Complete medical history review
- Baseline laboratory values
- Cardiovascular screening
- Allergy assessment
- Previous peptide exposure documentation
During-Study Monitoring
- Daily symptom tracking
- Weekly vital sign assessments
- Bi-weekly laboratory monitoring
- Monthly comprehensive evaluations
- Continuous adverse event documentation
Post-Study Follow-up
- Recovery period monitoring
- Long-term effect assessment
- Natural hormone recovery tracking
- Comprehensive final evaluation
Documentation Standards
Proper documentation of cjc1295/ipamorelin side effects serves multiple purposes:
- Individual Safety: Tracking personal response patterns
- Protocol Optimization: Identifying optimal dosing strategies
- Regulatory Compliance: Meeting research standards
- Future Research: Contributing to safety databases
- Risk Assessment: Evaluating overall safety profiles
Research facilities often develop standardized forms and tracking systems to ensure comprehensive documentation of all adverse events and side effects.
Emergency Response Protocols
Recognizing Serious Reactions
While severe cjc1295/ipamorelin side effects remain rare, research facilities must prepare for emergency situations:
Immediate Intervention Required:
- Difficulty breathing or swallowing
- Severe allergic reactions (anaphylaxis)
- Chest pain or severe cardiovascular symptoms
- Severe neurological symptoms
- Uncontrollable bleeding at injection sites
Monitoring Required:
- Persistent severe headaches
- Significant blood pressure changes
- Prolonged nausea or vomiting
- Unusual fatigue or weakness
- Skin reactions beyond injection sites
Response Procedures
Effective emergency response for serious cjc1295 ipamorelin side effects includes:
- Immediate Assessment: Vital signs and symptom severity
- Intervention Protocols: Predetermined treatment algorithms
- Medical Consultation: Access to qualified medical professionals
- Documentation: Comprehensive adverse event reporting
- Follow-up Care: Continued monitoring and support
Research facilities should maintain emergency supplies including antihistamines, epinephrine, and other emergency medications as appropriate for their research protocols.
Future Research Directions
Emerging Safety Data
The understanding of cjc1295/ipamorelin side effects continues to evolve as more research data becomes available:
Long-term Studies
Current research focuses on extended safety profiles, particularly:
- Multi-year exposure effects
- Recovery patterns after discontinuation
- Potential for cumulative toxicity
- Individual susceptibility factors
Mechanistic Research
Scientists are investigating the biological mechanisms behind side effects to:
- Predict individual risk factors
- Develop mitigation strategies
- Optimize dosing protocols
- Identify contraindications
Population Studies
Broader research initiatives aim to:
- Establish population-wide safety profiles
- Identify genetic susceptibility markers
- Develop personalized dosing strategies
- Create comprehensive safety databases
Technology Integration
Modern research facilities are incorporating technology to better track and manage cjc1295 ipamorelin side effects:
Digital Monitoring Systems
- Real-time symptom tracking applications
- Automated vital sign monitoring
- Electronic adverse event reporting
- Predictive analytics for risk assessment
Laboratory Integration
- Automated laboratory result tracking
- Biomarker monitoring systems
- Hormone level trend analysis
- Comprehensive data integration platforms
These technological advances help researchers better understand and manage the safety profiles of peptide research protocols.
Conclusion
Understanding cjc1295/ipamorelin side effects represents a critical component of responsible peptide research. While this peptide combination generally demonstrates a favorable safety profile in research settings, proper awareness and management of potential adverse reactions ensure optimal research outcomes and participant safety.
The most common side effects—injection site reactions, flushing, headaches, and mild fatigue—typically remain manageable through proper protocols and dose optimization. However, researchers must remain vigilant for more serious reactions and maintain comprehensive safety monitoring throughout research protocols.
Key Action Steps for Researchers:
- Implement comprehensive safety protocols before initiating any CJC1295/Ipamorelin research
- Source high-quality, tested peptides from reputable suppliers to minimize quality-related adverse effects
- Develop robust monitoring systems for tracking and managing side effects throughout research protocols
- Establish emergency response procedures for managing serious adverse reactions
- Maintain detailed documentation of all side effects and adverse events for future reference
- Consider individual risk factors when developing personalized research protocols
- Stay current with emerging safety data and adjust protocols accordingly
The future of peptide research depends on maintaining the highest safety standards while advancing our understanding of these powerful compounds. By prioritizing safety and comprehensive monitoring, researchers can continue to explore the potential benefits of CJC1295/Ipamorelin combinations while minimizing risks to research participants.
For researchers seeking high-quality peptides with comprehensive testing and documentation, establishing relationships with reputable suppliers ensures access to the materials necessary for safe and effective research protocols. The continued advancement of peptide science relies on this foundation of safety, quality, and scientific rigor.
References
[1] Laboratory Safety Guidelines for Peptide Research. Journal of Research Safety, 2024.
SEO Meta Information:
Meta Title: CJC1295/Ipamorelin Side Effects Guide 2025 | Research Safety
Meta Description: Comprehensive guide to CJC1295/Ipamorelin side effects in research. Learn about common reactions, safety protocols, and risk management for peptide studies.
