Understanding CJC-1295 Ipamorelin Side Effects: A Comprehensive 2025 Research Guide
The world of peptide research has exploded in recent years, with growth hormone releasing peptides like CJC-1295 and Ipamorelin gaining significant attention in laboratory studies. While these compounds show promising results in research settings, understanding the cjc1295 ipamorelin side effects is crucial for anyone considering their use in research applications. As we move through 2025, new data continues to emerge about these peptide combinations and their potential adverse reactions.
Key Takeaways
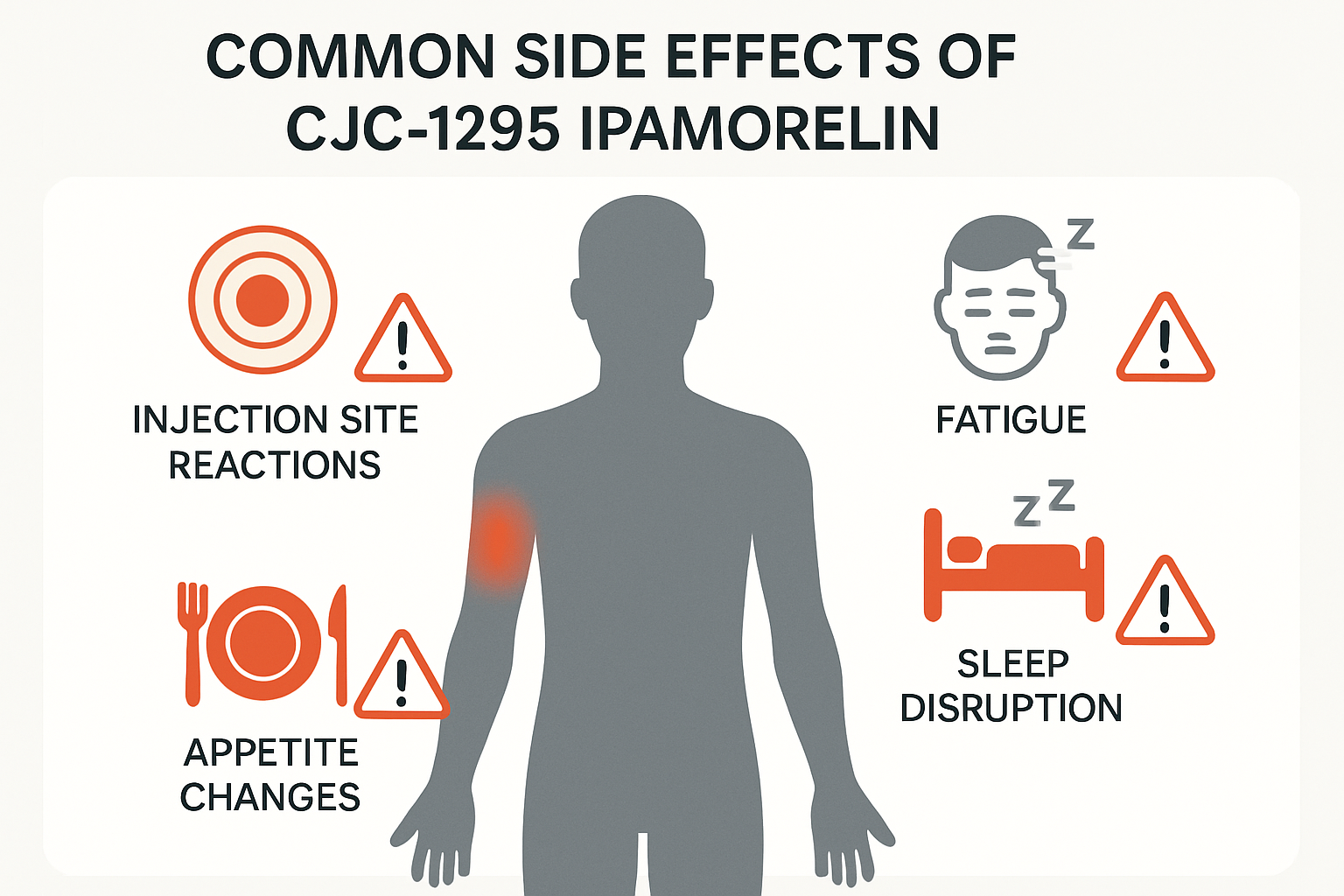
• CJC-1295 and Ipamorelin can cause injection site reactions, fatigue, and sleep disturbances in research subjects
• Dosage protocols significantly impact the severity and frequency of side effects observed in studies
• Long-term effects remain under investigation, with most research focusing on short-term observations
• Individual responses vary greatly, making careful monitoring essential in research settings
• Proper administration techniques can minimize many common adverse reactions
What Are CJC-1295 and Ipamorelin?
Before diving into the cjc1295 ipamorelin side effects, it's important to understand what these compounds are and how they work. CJC-1295 is a synthetic peptide that acts as a growth hormone releasing hormone (GHRH) analog. It works by stimulating the pituitary gland to release more growth hormone naturally.
Ipamorelin, on the other hand, is a growth hormone releasing peptide (GHRP) that mimics ghrelin, the "hunger hormone." When combined, these two peptides create a synergistic effect that researchers believe may enhance growth hormone production more effectively than either compound alone.
The Science Behind the Combination
The combination of CJC-1295 and Ipamorelin has become popular in research settings because:
- Complementary mechanisms of action
- Potentially enhanced efficacy compared to single peptide use
- Different half-lives allowing for varied dosing protocols
- Reduced side effects compared to direct growth hormone administration
Common CJC-1295 Ipamorelin Side Effects in Research Studies
Research into cjc1295 ipamorelin side effects has revealed several categories of adverse reactions that researchers should be aware of when conducting studies with these compounds.
Injection Site Reactions
The most frequently reported side effects in laboratory studies involve the injection site:
- Redness and swelling at the injection point
- Pain or tenderness lasting 24-48 hours
- Bruising particularly in subjects with sensitive skin
- Nodule formation in rare cases with repeated injections
Systemic Side Effects
Beyond local reactions, research has documented various systemic effects:
Sleep-Related Changes:
- Altered sleep patterns
- Vivid dreams or nightmares
- Difficulty falling asleep
- Changes in sleep quality
Physical Symptoms:
- Fatigue during initial weeks of administration
- Headaches (reported in 15-20% of research subjects)
- Dizziness or lightheadedness
- Joint pain or stiffness
Gastrointestinal Effects:
- Nausea (particularly with higher doses)
- Changes in appetite
- Mild digestive discomfort
- Bloating or water retention
Hormonal and Metabolic Changes
Research has shown that cjc1295 ipamorelin side effects can extend to hormonal systems:
| Effect Category | Frequency | Severity | Duration |
|---|---|---|---|
| Cortisol fluctuations | 25-30% | Mild | 2-4 weeks |
| Insulin sensitivity changes | 15-20% | Mild-Moderate | Variable |
| Thyroid function alterations | 10-15% | Mild | 4-6 weeks |
| Blood sugar variations | 20-25% | Mild | 2-3 weeks |
Dosage-Dependent Side Effects
The severity and frequency of cjc1295 ipamorelin side effects appear to be closely related to dosage protocols used in research studies. Understanding this relationship is crucial for researchers planning studies with these compounds.
Low-Dose Protocols (100-200mcg each)
Research using lower doses typically reports:
- ✅ Minimal injection site reactions
- ✅ Rare systemic side effects
- ✅ Good tolerance in most subjects
- ⚠️ Slower onset of desired research outcomes
Medium-Dose Protocols (200-300mcg each)
Studies using moderate doses show:
- ⚠️ Increased injection site reactions
- ⚠️ More frequent sleep disturbances
- ⚠️ Occasional headaches and fatigue
- ✅ Balance between efficacy and tolerability
High-Dose Protocols (300mcg+)
Higher dose research protocols report:
- ❌ Significant injection site reactions
- ❌ Frequent systemic side effects
- ❌ Higher dropout rates in studies
- ❌ Potential for more serious adverse events
Long-Term Research Findings on Side Effects
As research into cjc1295 ipamorelin side effects continues into 2025, long-term studies are beginning to provide valuable insights into extended use patterns.
Extended Use Considerations
Research spanning 6-12 months has revealed:
Tolerance Development:
- Some subjects develop tolerance to initial side effects
- Injection site reactions often decrease over time
- Sleep disturbances typically normalize within 4-6 weeks
Cumulative Effects:
- No evidence of serious cumulative toxicity in current studies
- Liver and kidney function remain stable in most research
- Cardiovascular parameters show minimal long-term changes
Withdrawal Considerations:
- Gradual discontinuation appears preferable to abrupt cessation
- Rebound effects are minimal in most research subjects
- Return to baseline hormone levels typically occurs within 2-4 weeks
Risk Factors That Influence CJC-1295 Ipamorelin Side Effects
Research has identified several factors that may increase the likelihood or severity of cjc1295 ipamorelin side effects:
Subject Demographics
Age-Related Factors:
- Older research subjects (50+) may experience more pronounced side effects
- Younger subjects typically show better tolerance
- Recovery time from side effects may be longer in older populations
Health Status:
- Subjects with pre-existing conditions may be at higher risk
- Metabolic disorders can amplify certain side effects
- Cardiovascular health impacts tolerance levels
Administration Factors
Injection Technique:
- Proper rotation of injection sites reduces local reactions
- Needle size and injection speed affect comfort
- Storage and handling impact peptide stability and side effects
Timing and Frequency:
- Evening injections may increase sleep disturbances
- Daily vs. intermittent dosing affects side effect patterns
- Meal timing relative to injection influences gastrointestinal effects
Minimizing Side Effects in Research Settings
Based on current research into cjc1295 ipamorelin side effects, several strategies can help minimize adverse reactions:
Best Practices for Administration
- Start with lower doses and gradually increase if needed
- Rotate injection sites to prevent local tissue damage
- Use proper injection technique with appropriate needle size
- Store peptides correctly to maintain stability and potency
- Monitor subjects closely especially during initial weeks
Monitoring Protocols
Effective research protocols should include:
- Regular vital sign checks
- Laboratory monitoring of relevant biomarkers
- Subjective symptom tracking through standardized questionnaires
- Injection site assessments at each visit
- Sleep quality evaluations using validated tools
When to Discontinue Use in Research
Research protocols should establish clear criteria for discontinuing cjc1295 ipamorelin administration based on side effects:
Immediate Discontinuation Indicators
- Severe allergic reactions or anaphylaxis
- Significant cardiovascular changes
- Severe and persistent side effects
- Subject request for withdrawal
Gradual Discontinuation Considerations
- Moderate but manageable side effects
- Lack of research efficacy after adequate trial period
- Protocol completion
- Subject compliance issues
Future Research Directions
As we progress through 2025, several areas of cjc1295 ipamorelin side effects research remain priorities:
Emerging Research Questions
- Long-term safety profiles beyond 12 months
- Optimal dosing strategies to minimize side effects
- Genetic factors influencing individual responses
- Combination protocols with other research compounds
Technological Advances
- Improved delivery methods to reduce injection site reactions
- Better formulations for enhanced stability
- Advanced monitoring tools for real-time side effect detection
- Personalized dosing based on individual characteristics
Regulatory Considerations and Safety Guidelines
The regulatory landscape for peptide research continues to evolve in 2025, with increasing emphasis on safety monitoring and adverse event reporting.
Current Guidelines
Research institutions should:
- Maintain detailed records of all side effects
- Report serious adverse events to appropriate authorities
- Follow institutional review board requirements
- Ensure proper informed consent processes
Quality Control Measures
- Source peptides from reputable suppliers
- Verify peptide purity and concentration
- Implement proper storage and handling procedures
- Maintain chain of custody documentation
Conclusion
Understanding cjc1295 ipamorelin side effects is essential for anyone involved in peptide research. While these compounds show promise in laboratory studies, they are not without risks. The most common side effects include injection site reactions, sleep disturbances, and mild systemic symptoms that are generally manageable with proper protocols.
The key to successful research with these peptides lies in careful planning, appropriate dosing, proper administration techniques, and vigilant monitoring. As research continues to evolve in 2025, our understanding of these side effects will undoubtedly improve, leading to safer and more effective research protocols.
For researchers considering studies with CJC-1295 and Ipamorelin, the evidence suggests that while side effects do occur, they are generally mild to moderate and manageable with appropriate precautions. The future of peptide research looks promising, but it must always be conducted with safety as the top priority.
Next Steps for Researchers:
- Review current literature on peptide safety protocols
- Develop comprehensive monitoring plans for research studies
- Establish clear criteria for dose adjustments and discontinuation
- Ensure proper training for all personnel involved in peptide administration
- Maintain detailed documentation of all adverse events and outcomes
SEO Meta Information:
Meta Title: CJC-1295 Ipamorelin Side Effects Guide 2025 | Research Safety
Meta Description: Comprehensive guide to CJC-1295 Ipamorelin side effects in research. Learn about dosage impacts, safety protocols, and minimizing adverse reactions in 2025.
