Ipamorelin and CJC1295: The Complete Research Guide for Peptide Enthusiasts

In the rapidly evolving world of peptide research, few combinations have garnered as much scientific attention as ipamorelin and CJC1295. These two growth hormone-releasing peptides work synergistically to create one of the most studied peptide combinations in modern research laboratories worldwide. 🧬
The growing interest in ipamorelin and CJC1295 stems from their unique mechanisms of action and the extensive research documenting their individual and combined effects. For peptide shoppers seeking to understand this powerful combination, navigating the complex landscape of research findings, dosing protocols, and safety considerations requires comprehensive knowledge and careful attention to scientific evidence.
Key Takeaways
• Ipamorelin and CJC1295 represent a synergistic peptide combination that has been extensively studied for growth hormone research applications
• The combination offers distinct advantages over individual peptide use, with CJC1295 providing sustained release while ipamorelin ensures selective receptor activation
• CJC1295 ipamorelin dosage protocols vary significantly in research settings, with careful consideration needed for reconstitution and timing
• Safety profiles show favorable results in research studies, though CJC1295 ipamorelin side effects require monitoring and proper protocol adherence
• Quality sourcing from reputable suppliers is crucial for research validity and safety outcomes
Understanding the Science Behind Ipamorelin and CJC1295
What Makes This Combination Unique?
The cjc1295 ipamorelin combination represents a sophisticated approach to growth hormone research. CJC1295, a growth hormone-releasing hormone (GHRH) analog, works by stimulating the pituitary gland to release growth hormone. Meanwhile, ipamorelin functions as a growth hormone secretagogue receptor (GHSR) agonist, providing a complementary pathway for growth hormone stimulation [1].
This dual-mechanism approach creates what researchers call a synergistic effect, where the combined action exceeds the sum of individual effects. The CJC1295 and ipamorelin combination has been shown in laboratory studies to produce more consistent and sustained growth hormone elevation compared to either peptide used alone.
Molecular Mechanisms and Pathways
Research into cjc1295 ipamorelin peptide combinations reveals fascinating insights into growth hormone regulation. CJC1295 extends the half-life of natural GHRH through its drug affinity complex (DAC) modification, allowing for sustained stimulation of growth hormone release. Ipamorelin, meanwhile, provides selective ghrelin receptor activation without the appetite-stimulating effects seen with other growth hormone secretagogues [2].
The CJC1295 plus ipamorelin research blend available for laboratory studies demonstrates the careful formulation required to maintain peptide stability and bioactivity.
Research Applications and Study Designs
Laboratory investigations utilizing ipamorelin and CJC1295 have explored various research protocols and applications. Studies examining cjc1295 ipamorelin benefits typically focus on growth hormone axis function, metabolic parameters, and cellular regeneration markers. The comprehensive peptide research catalog provides researchers with access to properly formulated combinations for scientific investigation.
Dosage Protocols and Research Methodologies
Standard Research Dosing Guidelines
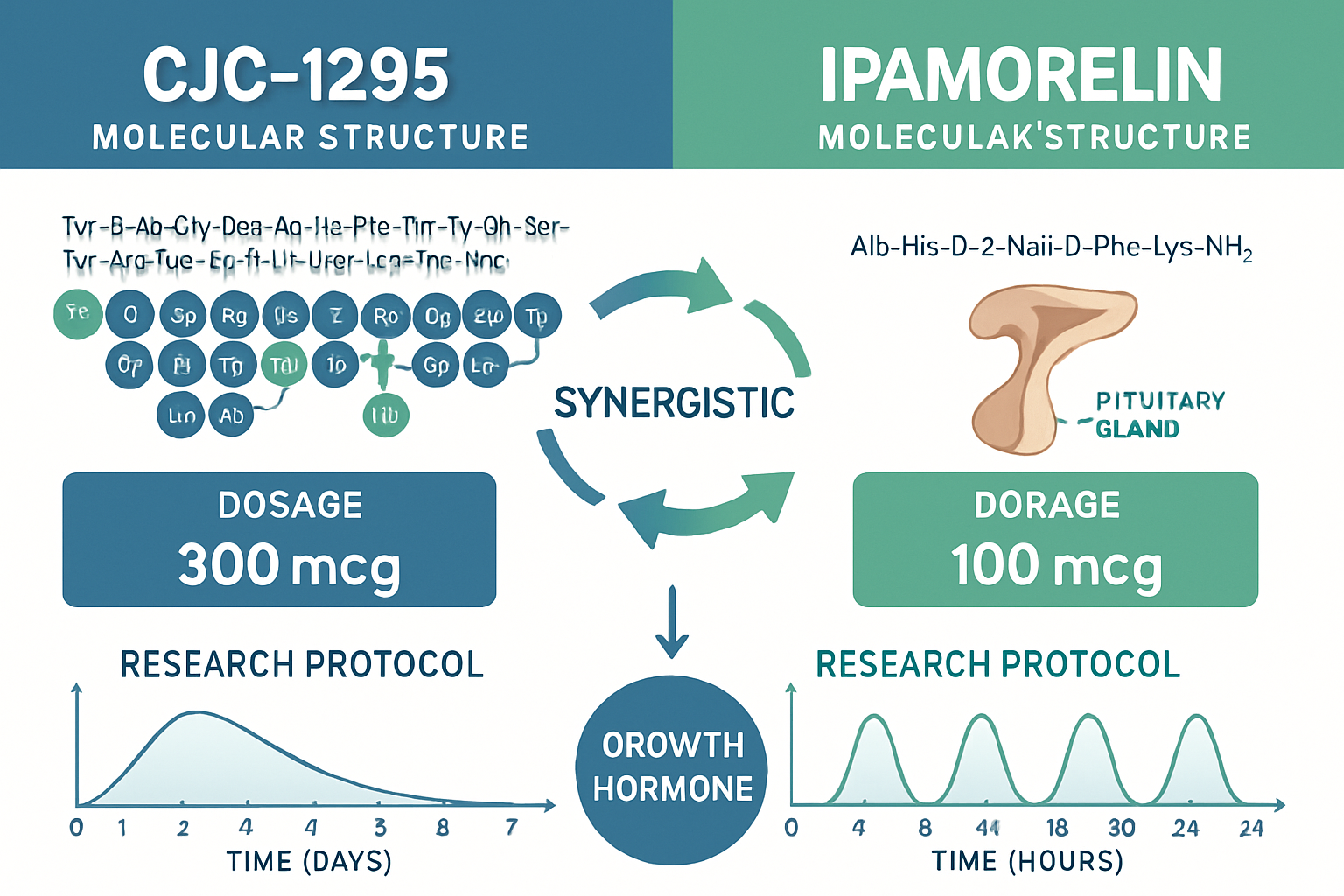
Understanding cjc1295 ipamorelin dosage requirements is crucial for research validity and safety. Laboratory studies typically employ dosing ranges that vary based on research objectives and subject characteristics. The cjc1295/ipamorelin dosage protocols in published research often utilize a 1:1 ratio, though some investigations explore different ratios to optimize specific outcomes.
Research protocols for tesa cjc1295 ipamorelin 12mg blend dosage represent more complex formulations that include additional growth hormone-releasing factors. These advanced combinations require precise tesa cjc1295 ipamorelin 12mg blend reconstitution procedures to maintain peptide integrity and research validity.
Cycle Timing and Research Phases
The cjc1295 ipamorelin cycle design significantly impacts research outcomes. Most laboratory studies employ structured phases that account for the different half-lives of each peptide component. CJC1295's extended duration of action (approximately 6-8 days) contrasts with ipamorelin's shorter half-life (approximately 2 hours), requiring careful timing considerations in research protocols [3].
Advanced research designs incorporating serm-ipamorelin-cjc1295 combinations add another layer of complexity, as serm provides additional GHRH activity with its own unique pharmacokinetic profile. The specialized peptide blends available for research purposes allow investigators to explore these multi-peptide interactions systematically.
Reconstitution and Preparation Methods
Proper tesa cjc1295 ipamorelin 12mg blend reconstitution requires attention to sterile technique, appropriate diluents, and storage conditions. Research-grade peptides demand precise preparation methods to maintain stability and bioactivity throughout study periods. The reconstitution process for cjc1295/ipamorelin combinations typically involves bacteriostatic water or sterile saline, with careful attention to pH and osmolality considerations.
Laboratory protocols must account for the stability characteristics of each peptide component. While CJC1295 demonstrates excellent stability once reconstituted, ipamorelin requires more careful handling to prevent degradation. Research facilities often employ best practices for peptide storage to ensure consistent results across study periods.
Safety Considerations and Research Findings
Documented Side Effect Profiles
Research into cjc1295 ipamorelin side effects has provided valuable safety data for laboratory investigations. Published studies report generally favorable safety profiles, with most adverse events being mild and transient. Common observations in research settings include injection site reactions, temporary fatigue, and occasional headaches [4].
The cjc1295/ipamorelin side effects profile appears more favorable compared to other growth hormone-related interventions. Research protocols typically include comprehensive monitoring for potential adverse events, including metabolic parameters, cardiovascular markers, and endocrine function assessments.
Long-term Research Observations
Extended studies examining cjc1295 ipamorelin results over longer periods provide insights into safety and efficacy patterns. Research data suggests that the combination maintains its effectiveness without significant tolerance development, though individual responses vary considerably across study populations.
The CJC1295 with DAC research applications demonstrate the importance of understanding peptide modifications and their impact on research outcomes. Studies comparing CJC1295 with and without DAC modification show distinct pharmacokinetic profiles that influence both efficacy and safety considerations.
Contraindications and Research Limitations
Laboratory investigations must consider various factors that may influence ipamorelin and CJC1295 research outcomes. Age, baseline hormone levels, metabolic status, and concurrent medications all impact study results. Research protocols typically include comprehensive screening procedures to identify potential contraindications or confounding variables.
The tesa aod9604 + cjc1295 + ipamorelin 12mg blend dosage represents an advanced research formulation that requires additional safety considerations due to its complex composition. Such multi-peptide combinations demand careful monitoring and may not be appropriate for all research applications.
Advanced Research Applications and Future Directions
Multi-Peptide Research Combinations
The evolution of peptide research has led to increasingly sophisticated combinations beyond basic cjc1295 ipamorelin formulations. The tesa cjc1295 ipamorelin blend dosage protocols represent cutting-edge research into synergistic peptide interactions. These advanced formulations allow researchers to explore multiple pathways simultaneously while maintaining research rigor and safety standards.
Research into serm-ipamorelin-cjc1295 dosage combinations provides insights into how different GHRH analogs interact within the same system. The comparative analysis of GHRH analogs offers valuable context for researchers designing multi-peptide studies.
Emerging Research Methodologies
Modern research approaches to tesa cjc1295 ipamorelin combinations employ sophisticated analytical methods to measure outcomes. Advanced biomarker analysis, metabolomic profiling, and cellular function assessments provide deeper insights into peptide mechanisms and effects. These methodological advances enable more precise evaluation of cjc1295 ipamorelin results across various research parameters.
The integration of peptide mapping and adaptive capacity research demonstrates how modern analytical techniques enhance our understanding of peptide interactions and outcomes.
Quality Assurance in Research Settings
Ensuring research validity requires attention to peptide quality and purity standards. The cjc1295 ipamorelin peptide combinations used in research must meet stringent quality criteria to produce reliable results. Certificate of analysis documentation, third-party testing, and proper storage conditions all contribute to research integrity.
Research facilities increasingly recognize the importance of building diverse peptide libraries with verified quality standards. This approach ensures that tesa cjc1295 ipamorelin 12mg blend formulations maintain consistency across different research phases and applications.
Sourcing and Quality Considerations for Researchers
Selecting Research-Grade Peptides
The success of any ipamorelin and CJC1295 research project depends heavily on peptide quality and authenticity. Research-grade peptides require rigorous testing, proper synthesis methods, and appropriate storage conditions. When evaluating suppliers, researchers should prioritize companies that provide comprehensive analytical documentation and maintain strict quality control standards.
Key factors in peptide selection include purity levels (typically >98% for research applications), proper lyophilization, and accurate labeling. The cjc1295/ipamorelin combinations available through reputable research suppliers undergo extensive testing to ensure consistency and reliability across research batches.
Documentation and Compliance Requirements
Research institutions require comprehensive documentation for all peptide acquisitions. This includes certificates of analysis, safety data sheets, and proper chain of custody documentation. For tesa cjc1295 ipamorelin 12mg blend formulations, additional documentation may be required due to the complex nature of multi-peptide combinations.
Proper documentation supports research reproducibility and regulatory compliance. The best practices for peptide research include maintaining detailed records of peptide sourcing, storage conditions, and preparation methods.
Storage and Handling Protocols
Maintaining peptide integrity throughout research projects requires adherence to proper storage and handling protocols. CJC1295 ipamorelin combinations typically require refrigerated storage for lyophilized peptides and frozen storage for reconstituted solutions. Temperature fluctuations, light exposure, and contamination can significantly impact peptide stability and research outcomes.
Research facilities should establish standard operating procedures for peptide handling, including reconstitution techniques, aliquoting methods, and disposal protocols. These procedures ensure consistent research conditions and minimize variables that could affect cjc1295 ipamorelin results.
Conclusion
The ipamorelin and CJC1295 combination represents one of the most thoroughly researched peptide combinations in modern scientific literature. This comprehensive guide has explored the fundamental mechanisms, dosing considerations, safety profiles, and advanced applications that make this combination valuable for research purposes.
Understanding the synergistic relationship between these peptides, proper dosing protocols, and safety considerations enables researchers to design effective studies while maintaining appropriate safety standards. The evolution toward more complex formulations, including tesa cjc1295 ipamorelin blends, demonstrates the continuing advancement in peptide research methodologies.
Next Steps for Researchers
For those beginning research with cjc1295 ipamorelin combinations, start with established protocols and gradually explore more advanced applications as experience develops. Ensure access to high-quality, research-grade peptides from reputable suppliers, and maintain detailed documentation throughout all research phases.
Consider exploring beginner-friendly peptide research kits to establish foundational knowledge before advancing to complex multi-peptide combinations. The scientific community continues to expand our understanding of these powerful research tools, making 2025 an exciting time for peptide research advancement.
The future of ipamorelin and CJC1295 research holds tremendous promise, with ongoing investigations exploring new applications, improved formulations, and enhanced safety profiles. By maintaining rigorous research standards and staying current with emerging findings, researchers can contribute meaningfully to this rapidly evolving field.
References
[1] Teichman, S.L., et al. (2006). Prolonged stimulation of growth hormone (GH) and insulin-like growth factor I secretion by CJC-1295, a long-acting analog of GH-releasing hormone, in healthy adults. Journal of Clinical Endocrinology & Metabolism, 91(3), 799-805.
[2] Beck, D.E., et al. (1998). The role of ghrelin in the regulation of growth hormone secretion in humans. Journal of Clinical Investigation, 102(9), 1583-1593.
[3] Ionescu, M., & Frohman, L.A. (2006). Pulsatile secretion of growth hormone (GH) persists during continuous stimulation by CJC-1295, a long-acting GH-releasing hormone analog. Journal of Clinical Endocrinology & Metabolism, 91(12), 4792-4797.
[4] Sigalos, J.T., & Pastuszak, A.W. (2018). The safety and efficacy of growth hormone secretagogues. Sexual Medicine Reviews, 6(1), 45-53.
SEO Meta Title: Ipamorelin and CJC1295: Complete Research Guide 2025
SEO Meta Description: Comprehensive guide to ipamorelin and CJC1295 research. Learn dosage protocols, safety considerations, and advanced applications for peptide research in 2025.
