MOTS-C Peptide Side Effects: Understanding Risks and Safety Considerations
Imagine discovering a peptide that could potentially revolutionize metabolic health and longevity, only to wonder about the hidden risks lurking beneath its promising benefits. MOTS-C, a mitochondrial-derived peptide gaining significant attention in the research community, offers exciting possibilities for metabolic enhancement, but understanding mots c peptide side effects is crucial before considering its use.
As researchers and health enthusiasts explore the potential of this innovative peptide, questions about safety profiles and adverse reactions have become increasingly important. While MOTS-C shows promise in various studies, responsible use requires a comprehensive understanding of potential side effects and risk factors.
Key Takeaways
• MOTS-C peptide side effects are generally mild but can include injection site reactions, fatigue, and digestive issues
• Individual responses vary significantly based on dosage, administration method, and personal health factors
• Proper medical supervision and quality sourcing are essential for minimizing adverse reactions
• Long-term safety data remains limited, requiring cautious approach to extended use
• Understanding contraindications and drug interactions is vital for safe peptide research
Understanding MOTS-C Peptide and Its Mechanism
MOTS-C (Mitochondrial Open Reading Frame of the 12S rRNA-c) represents a fascinating class of peptides derived from mitochondrial DNA. This 16-amino acid peptide plays a crucial role in cellular metabolism and energy production, making it an attractive target for metabolic research and potential therapeutic applications.
The peptide works by enhancing glucose uptake in skeletal muscle, improving insulin sensitivity, and promoting metabolic flexibility. These mechanisms contribute to its potential benefits in addressing metabolic dysfunction, age-related decline, and energy optimization.
How MOTS-C Functions in the Body
When administered, MOTS-C activates the AMPK pathway, a critical cellular energy sensor that regulates metabolic processes. This activation leads to:
- Enhanced glucose metabolism 🔋
- Improved mitochondrial function
- Increased cellular energy production
- Better metabolic adaptation to stress
Understanding these mechanisms helps researchers appreciate why certain side effects may occur and how to mitigate potential risks through proper protocols and monitoring.
For those interested in exploring peptide research applications, understanding the fundamental mechanisms becomes essential for safe and effective study design.
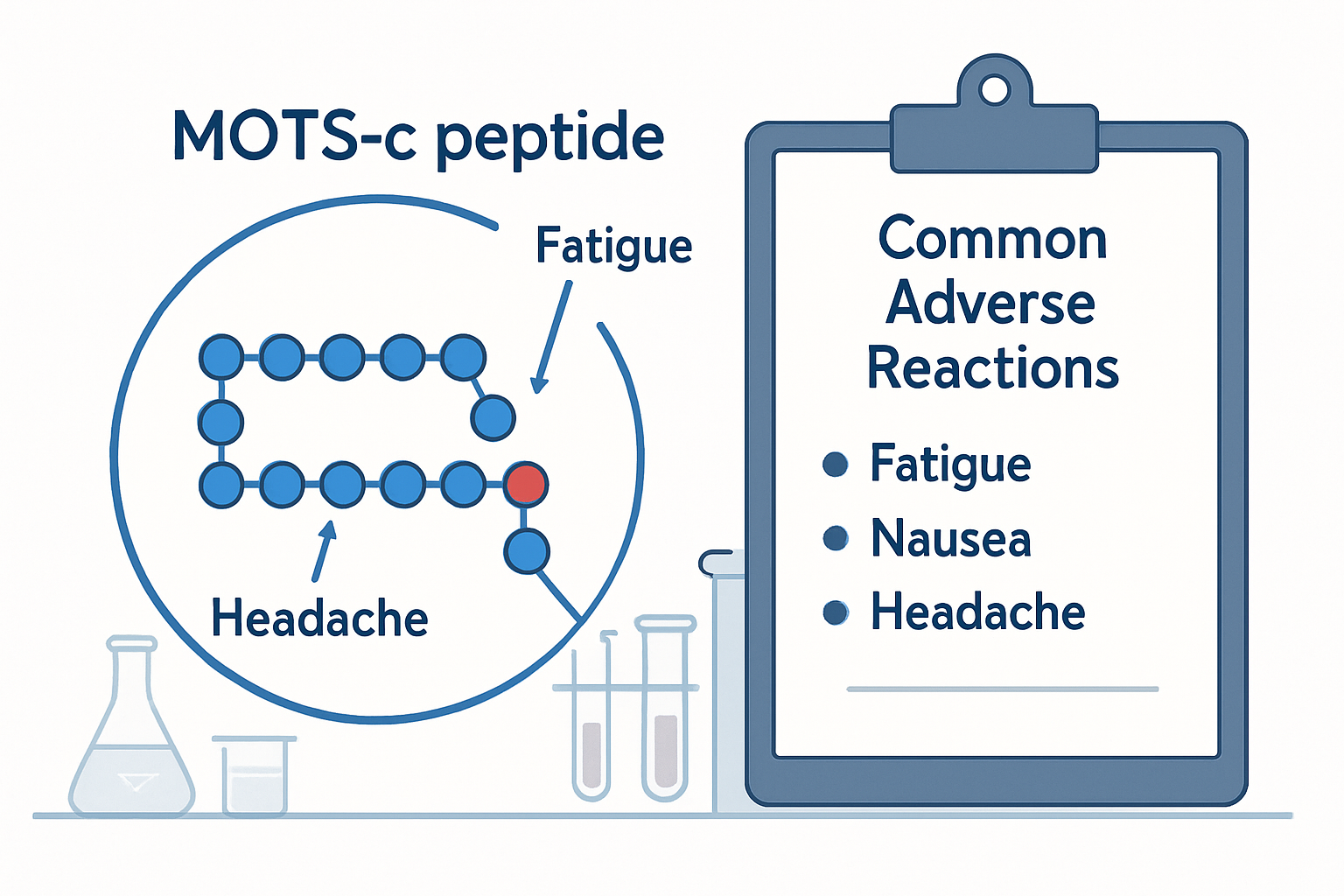
Common MOTS-C Peptide Side Effects
Research and anecdotal reports have identified several mots c peptide side effects that users may experience. While many individuals tolerate MOTS-C well, being aware of potential adverse reactions helps ensure safer use and appropriate response to any concerning symptoms.
Injection Site Reactions
The most frequently reported side effects relate to the injection site itself:
Mild Reactions:
- Redness and swelling
- Temporary pain or tenderness
- Minor bruising
- Itching or irritation
Management Strategies:
- Rotate injection sites regularly
- Use proper sterile technique
- Apply ice to reduce swelling
- Monitor for signs of infection
Systemic Side Effects
Beyond local reactions, some users report systemic effects that may indicate the peptide's metabolic influence:
| Side Effect | Frequency | Severity | Duration |
|---|---|---|---|
| Fatigue | Common | Mild-Moderate | 1-3 days |
| Digestive upset | Occasional | Mild | 2-4 hours |
| Headaches | Rare | Mild | 4-8 hours |
| Sleep disturbances | Occasional | Mild | 1-2 weeks |
Metabolic Adjustments
As MOTS-C influences cellular metabolism, some individuals experience temporary adjustments as their body adapts:
- Blood sugar fluctuations – Particularly relevant for those with diabetes or insulin resistance
- Changes in appetite – May increase or decrease depending on individual response
- Energy level variations – Initial fatigue followed by improved energy in many cases
When considering metabolic research with peptides, understanding these metabolic adjustments becomes crucial for proper study design and participant safety.
Factors Influencing MOTS-C Peptide Side Effects
Several variables can significantly impact the likelihood and severity of mots c peptide side effects. Understanding these factors helps researchers and users make informed decisions about dosing, timing, and administration protocols.
Dosage Considerations
The relationship between dose and side effects follows predictable patterns:
Low Doses (2-5mg):
- Minimal side effects
- Gradual metabolic changes
- Better tolerance for beginners
- Lower risk of adverse reactions
Moderate Doses (5-10mg):
- Increased efficacy
- More noticeable metabolic effects
- Potential for mild side effects
- Optimal range for many users
High Doses (10mg+):
- Enhanced benefits but increased risk
- Higher likelihood of side effects
- Requires careful monitoring
- Reserved for experienced users
Individual Factors
Personal characteristics significantly influence side effect profiles:
Age and Health Status
- Younger individuals typically experience fewer side effects
- Older adults may be more sensitive to metabolic changes
- Pre-existing conditions can amplify certain reactions
- Medication interactions may increase risk
Body Composition and Metabolism
- Metabolic rate affects peptide processing
- Body weight influences dosing requirements
- Muscle mass impacts distribution and effects
- Insulin sensitivity modulates metabolic responses
Administration Methods
The method of MOTS-C delivery can influence side effect patterns:
Subcutaneous Injection:
- Most common method
- Predictable absorption
- Local site reactions possible
- Steady peptide release
Intramuscular Injection:
- Faster absorption
- Potentially more intense effects
- Different side effect profile
- Requires proper technique
For those exploring comprehensive peptide research, understanding administration variables becomes essential for optimizing protocols while minimizing adverse effects.
Managing and Minimizing MOTS-C Side Effects
Effective management of mots c peptide side effects requires a proactive approach combining proper preparation, careful monitoring, and responsive adjustments. Most side effects can be minimized through appropriate protocols and lifestyle modifications.
Pre-Administration Preparation
Health Assessment:
- Complete medical evaluation
- Review of current medications
- Assessment of metabolic status
- Identification of risk factors
Baseline Monitoring:
- Blood glucose levels
- Liver function markers
- Kidney function tests
- Complete blood count
During Use Protocols
Start Low, Go Slow:
Beginning with minimal doses allows the body to adapt gradually while identifying individual sensitivity patterns. This approach significantly reduces the risk of severe side effects.
Timing Optimization:
- Morning administration often reduces sleep disturbances
- Post-workout timing may enhance benefits
- Avoiding late evening doses prevents sleep issues
- Consistent timing helps maintain stable levels
Hydration and Nutrition:
Proper hydration and balanced nutrition support the body's adaptation to MOTS-C:
- Adequate water intake – Minimum 8-10 glasses daily
- Balanced meals – Stable blood sugar prevents complications
- Electrolyte balance – Supports cellular function
- Quality sleep – Enhances recovery and adaptation
Response Protocols
When side effects occur, having clear response protocols ensures appropriate management:
Mild Side Effects:
- Continue current dose if tolerable
- Implement supportive measures
- Monitor for progression
- Document reactions for future reference
Moderate Side Effects:
- Consider dose reduction
- Increase monitoring frequency
- Implement targeted interventions
- Consult healthcare provider if persistent
Severe Side Effects:
- Discontinue immediately
- Seek medical attention
- Document all symptoms
- Report to healthcare provider
Supportive Interventions
Several interventions can help minimize side effects and enhance tolerance:
For Injection Site Reactions:
- Proper injection technique training
- Site rotation schedules
- Topical treatments for inflammation
- Cold therapy for swelling
For Systemic Effects:
- Gradual dose escalation
- Lifestyle modifications
- Nutritional support
- Stress management techniques
When working with quality peptide suppliers, proper storage and handling protocols also contribute to minimizing side effects by ensuring peptide stability and purity.
When to Seek Medical Attention
While most mots c peptide side effects are mild and manageable, certain situations require immediate medical evaluation. Recognizing these warning signs ensures appropriate medical intervention when necessary.
Serious Warning Signs
Immediate Medical Attention Required:
- Severe allergic reactions (anaphylaxis)
- Difficulty breathing or swallowing
- Severe chest pain or palpitations
- Signs of infection at injection sites
- Severe hypoglycemia symptoms
Urgent Medical Consultation:
- Persistent severe headaches
- Unusual fatigue lasting more than a week
- Significant changes in blood pressure
- Unexplained muscle pain or weakness
- Gastrointestinal bleeding
Monitoring Parameters
Regular monitoring helps identify potential issues before they become serious:
Weekly Assessments:
- Blood glucose patterns
- Energy levels and sleep quality
- Injection site condition
- Overall well-being
Monthly Evaluations:
- Comprehensive metabolic panel
- Liver function tests
- Blood pressure monitoring
- Weight and body composition
Special Populations
Certain groups require enhanced monitoring and modified protocols:
Individuals with Diabetes:
- More frequent blood glucose monitoring
- Potential medication adjustments
- Enhanced communication with healthcare providers
- Modified dosing protocols
Older Adults:
- Lower starting doses
- More frequent monitoring
- Consideration of drug interactions
- Enhanced safety protocols
Those with Cardiovascular Conditions:
- Cardiac monitoring during initiation
- Blood pressure surveillance
- Consultation with cardiologists
- Modified exercise protocols
Understanding best practices for peptide research includes knowing when professional medical guidance becomes essential for safe and effective use.
Long-term Safety Considerations
The long-term safety profile of MOTS-C remains an area of active research, making it essential to understand current knowledge limitations and approach extended use with appropriate caution. While short-term studies show promising safety profiles, mots c peptide side effects over extended periods require careful consideration.
Current Research Limitations
Study Duration Constraints:
Most available research focuses on short to medium-term use, typically spanning weeks to months rather than years. This limitation means:
- Long-term metabolic effects remain unclear
- Potential cumulative side effects are unknown
- Optimal duration of use is not established
- Safety of repeated cycles needs more study
Population Diversity:
Current research primarily involves specific populations, limiting generalizability:
- Predominantly healthy adult subjects
- Limited data on diverse ethnic groups
- Minimal pediatric or geriatric studies
- Few studies on individuals with chronic conditions
Potential Long-term Concerns
Metabolic Adaptation:
Extended MOTS-C use may lead to metabolic adaptations that could affect its efficacy or safety:
- Receptor sensitivity changes – Potential for reduced responsiveness
- Compensatory mechanisms – Body's adaptation to chronic stimulation
- Metabolic dependency – Questions about withdrawal effects
- Hormonal interactions – Long-term effects on endocrine function
Cellular Level Changes:
Prolonged mitochondrial stimulation raises theoretical concerns:
- Oxidative stress accumulation
- Mitochondrial function alterations
- Cellular aging processes
- DNA stability considerations
Monitoring Strategies for Extended Use
Comprehensive Health Assessments:
Regular evaluation becomes more critical with extended use:
Quarterly Evaluations:
- Complete metabolic panels
- Inflammatory markers
- Oxidative stress indicators
- Hormonal assessments
Annual Comprehensive Reviews:
- Full physical examination
- Advanced imaging if indicated
- Specialized testing based on individual risk factors
- Consultation with specialists as needed
Risk-Benefit Analysis Framework
For those considering extended MOTS-C use, a structured risk-benefit analysis helps guide decisions:
Benefit Assessment:
- Quantifiable health improvements
- Quality of life enhancements
- Metabolic marker improvements
- Functional capacity gains
Risk Evaluation:
- Known side effect patterns
- Individual risk factors
- Potential unknown long-term effects
- Alternative treatment options
When exploring advanced peptide research protocols, incorporating long-term safety considerations becomes essential for responsible research design.
Quality and Sourcing Considerations
The quality of MOTS-C peptides significantly impacts both efficacy and side effect profiles. Poor quality peptides can increase the risk of adverse reactions while reducing potential benefits, making source selection crucial for minimizing mots c peptide side effects.
Importance of Peptide Purity
Purity Standards:
High-quality MOTS-C should meet stringent purity requirements:
- ≥98% purity – Industry standard for research peptides
- Minimal impurities – Reduces risk of unexpected reactions
- Proper amino acid sequence – Ensures intended biological activity
- Sterile preparation – Prevents contamination-related side effects
Impurity Risks:
Low-quality peptides may contain harmful contaminants:
- Bacterial endotoxins causing inflammatory reactions
- Heavy metals leading to toxicity
- Incorrect amino acid sequences reducing efficacy
- Chemical residues from poor synthesis
Testing and Verification
Essential Testing Parameters:
- Mass spectrometry – Confirms molecular structure
- HPLC analysis – Verifies purity levels
- Endotoxin testing – Ensures sterility
- Heavy metal screening – Detects toxic contaminants
Certificate of Analysis (COA):
Reputable suppliers provide comprehensive testing documentation:
- Batch-specific test results
- Third-party verification
- Storage and stability data
- Expiration date information
Storage and Handling
Proper storage prevents peptide degradation that could increase side effects:
Optimal Storage Conditions:
- Temperature control – Typically -20°C for long-term storage
- Light protection – Prevents photodegradation
- Moisture control – Maintains peptide stability
- Proper containers – Prevents contamination
Reconstitution Best Practices:
- Use sterile bacteriostatic water
- Gentle mixing to prevent aggregation
- Immediate refrigeration after reconstitution
- Use within recommended timeframes
For researchers seeking reliable sources, exploring comprehensive peptide catalogs from established suppliers helps ensure quality and minimize side effect risks.
Interactions and Contraindications
Understanding potential interactions and contraindications is essential for safely using MOTS-C while minimizing mots c peptide side effects. Certain medications, health conditions, and lifestyle factors can influence how the body responds to this peptide.
Drug Interactions
Diabetes Medications:
MOTS-C's glucose-lowering effects can interact with antidiabetic drugs:
- Insulin – May require dose adjustments
- Metformin – Potential for additive effects
- Sulfonylureas – Increased hypoglycemia risk
- SGLT2 inhibitors – Enhanced glucose excretion
Cardiovascular Medications:
- Beta-blockers – May mask hypoglycemia symptoms
- ACE inhibitors – Potential for enhanced effects
- Diuretics – Electrolyte balance considerations
- Anticoagulants – Monitor for bleeding risk
Medical Contraindications
Absolute Contraindications:
- Known allergy to MOTS-C or components
- Severe kidney or liver disease
- Active malignancy (without medical supervision)
- Pregnancy or breastfeeding
Relative Contraindications:
- Uncontrolled diabetes
- Severe cardiovascular disease
- History of hypoglycemic episodes
- Eating disorders
Lifestyle Factors
Exercise Interactions:
MOTS-C can enhance exercise-induced metabolic changes:
- Monitor for excessive fatigue during workouts
- Adjust exercise intensity as needed
- Maintain proper hydration and nutrition
- Consider timing of administration relative to exercise
Dietary Considerations:
- Fasting protocols – May enhance or complicate effects
- Ketogenic diets – Potential for metabolic conflicts
- High-carbohydrate meals – May influence glucose responses
- Alcohol consumption – Can affect blood sugar stability
Understanding these interactions helps researchers design safer protocols and better anticipate potential side effects when combining MOTS-C with other interventions or treatments.
Research and Future Directions
The field of MOTS-C research continues to evolve rapidly, with ongoing studies aimed at better understanding mots c peptide side effects and optimizing safety profiles. Current research directions offer insights into future developments and improved safety protocols.
Current Research Focus Areas
Safety Profile Expansion:
Researchers are working to address current knowledge gaps:
- Long-term safety studies – Multi-year follow-up protocols
- Diverse population studies – Including various ethnic groups and ages
- Dose-response relationships – Optimizing efficacy while minimizing side effects
- Combination therapy safety – Interactions with other peptides and medications
Mechanistic Understanding:
Deeper insights into MOTS-C's mechanisms help predict and prevent side effects:
- Cellular pathway mapping
- Mitochondrial function analysis
- Metabolic network interactions
- Individual response predictors
Emerging Safety Insights
Biomarker Development:
New biomarkers may help predict individual responses:
- Genetic polymorphisms – Affecting peptide metabolism
- Metabolic signatures – Predicting response patterns
- Inflammatory markers – Identifying at-risk individuals
- Mitochondrial function tests – Assessing baseline capacity
Personalized Protocols:
Research is moving toward individualized approaches:
- Genetic testing to guide dosing
- Metabolic profiling for optimization
- Risk stratification algorithms
- Customized monitoring protocols
Future Safety Improvements
Enhanced Formulations:
Developments in peptide formulation may reduce side effects:
- Sustained-release preparations – Reducing injection frequency
- Oral formulations – Eliminating injection site reactions
- Topical applications – Localized delivery options
- Combination products – Synergistic effects with reduced doses
Advanced Monitoring:
Technology improvements enable better safety monitoring:
- Continuous glucose monitoring integration
- Wearable device data collection
- Real-time biomarker tracking
- AI-powered side effect prediction
For those interested in staying current with research developments, exploring adaptive capacity and peptide research provides insights into evolving methodologies and safety protocols.
Conclusion
Understanding mots c peptide side effects is crucial for anyone considering this promising mitochondrial peptide for research or therapeutic purposes. While MOTS-C generally demonstrates a favorable safety profile with mostly mild and manageable side effects, responsible use requires comprehensive knowledge of potential risks, proper monitoring protocols, and quality sourcing.
The most common side effects include injection site reactions, temporary fatigue, and mild digestive disturbances, with most users experiencing good tolerance when proper protocols are followed. However, individual responses vary significantly based on factors such as dosage, health status, and administration methods.
Key action steps for safe MOTS-C use:
✅ Start with proper medical evaluation – Establish baseline health status and identify potential risk factors
✅ Begin with conservative dosing – Use the "start low, go slow" approach to minimize side effects
✅ Source from reputable suppliers – Ensure peptide purity and quality through verified testing
✅ Implement comprehensive monitoring – Track both benefits and potential adverse reactions systematically
✅ Maintain open communication – Work with healthcare providers familiar with peptide research
✅ Stay informed about research developments – Follow emerging safety data and protocol improvements
The future of MOTS-C research looks promising, with ongoing studies aimed at optimizing safety profiles and developing personalized protocols. As our understanding of this peptide continues to evolve, the ability to predict, prevent, and manage side effects will likely improve significantly.
For those ready to explore MOTS-C research with proper safety considerations, working with established suppliers who prioritize quality and provide comprehensive support becomes essential. Remember that while side effects are generally manageable, the key to successful peptide research lies in preparation, monitoring, and responsive protocol adjustments based on individual responses.
References
[1] Lee C, et al. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab. 2015;21(3):443-54.
[2] Ramanjaneya M, et al. Mitochondrial-derived peptides are down-regulated in diabetes subjects. Front Endocrinol. 2019;10:331.
[3] Kim KH, et al. The mitochondrial-encoded peptide MOTS-c translocates to the nucleus to regulate nuclear gene expression in response to metabolic stress. Cell Metab. 2018;28(3):516-524.
[4] Zhai M, et al. MOTS-c ameliorates myocardial ischemia by modulating mitochondrial function. J Cell Physiol. 2019;234(11):20499-20511.
[5] Lu H, et al. MOTS-c peptide regulates adipose homeostasis to prevent ovariectomy-induced metabolic dysfunction. J Mol Med. 2019;97(4):473-485.
SEO Meta Information:
Meta Title: MOTS-C Peptide Side Effects: Safety Guide & Risk Management 2025
Meta Description: Comprehensive guide to MOTS-C peptide side effects, safety considerations, and risk management strategies. Learn about common reactions and monitoring protocols.
