The Complete Guide to Selank Peptide Dosage: Research-Based Protocols and Administration Methods

Imagine having access to a synthetic peptide that could potentially enhance cognitive function and reduce anxiety without the dependency risks associated with traditional pharmaceuticals. Selank peptide dosage protocols have been extensively studied in clinical research, revealing fascinating insights about this unique heptapeptide's therapeutic potential and optimal administration methods.
Key Takeaways
• Standard dosage ranges: Clinical research shows effective Selank peptide dosage typically falls between 250-3000 mcg per day, divided into multiple administrations
• Preferred delivery method: Intranasal administration demonstrates 60-70% bioavailability, making it more efficient than other routes
• Treatment duration: Most studies utilize 14-28 day protocols, with some extending to 3 months for chronic conditions
• Multiple daily doses required: Due to Selank's 20-30 minute half-life, researchers typically administer doses 2-3 times daily
• Dose-dependent effects: Lower doses (250-400 mcg) show anxiolytic properties, while higher doses (600-3000 mcg) demonstrate enhanced cognitive benefits
Understanding Selank Peptide Fundamentals
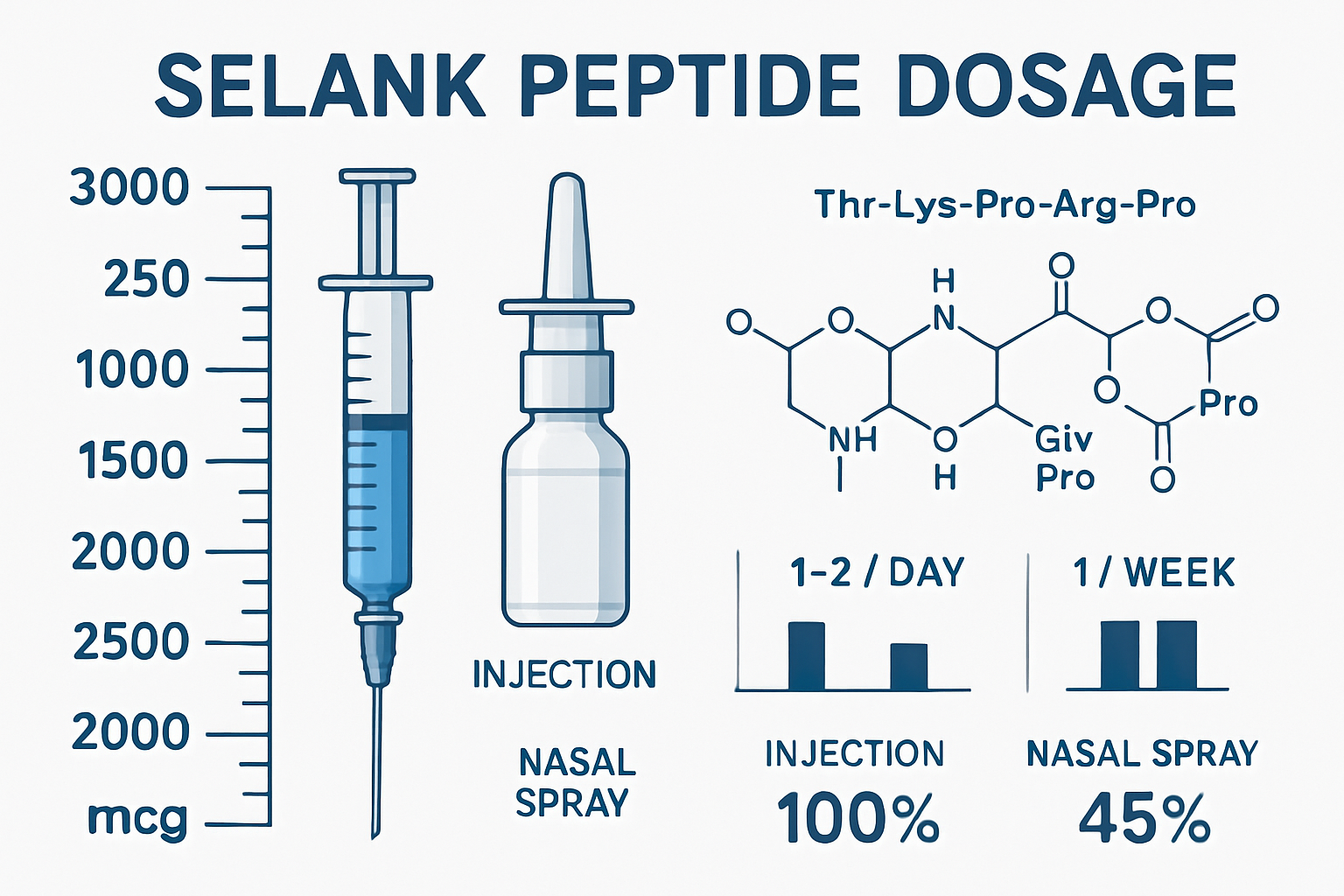
Selank represents a breakthrough in peptide research, developed by the Institute of Molecular Genetics of the Russian Academy of Sciences. This synthetic heptapeptide features the amino acid sequence Thr-Lys-Pro-Arg-Pro-Gly-Pro and has garnered significant attention for its unique properties in laboratory studies [1].
Unlike traditional anxiolytic compounds, Selank demonstrates remarkable stability and bioactivity in research applications. The peptide's molecular structure allows it to interact with specific receptor pathways while maintaining a favorable safety profile in clinical investigations.
Research indicates that Selank's mechanism of action involves modulation of neurotransmitter systems, particularly those related to GABA and serotonin pathways. This unique approach distinguishes it from conventional pharmaceuticals and makes it an intriguing subject for peptide research applications.
The peptide's development stemmed from efforts to create more effective therapeutic agents with reduced side effect profiles. Clinical researchers have extensively documented its pharmacokinetic properties, revealing important insights about optimal dosing strategies and administration protocols.
Understanding these fundamental characteristics becomes crucial when examining Selank peptide dosage protocols, as the compound's unique properties directly influence how researchers approach dosing and administration in laboratory settings.
Selank Peptide Dosage Protocols: Clinical Research Findings
Standard Dosage Ranges in Research
Clinical studies have established that effective Selank peptide dosage typically ranges from 250 mcg to 3000 mcg per day, with most research protocols utilizing doses within the 400-1800 mcg daily range [2]. These dosages have been extensively tested across multiple clinical trials, providing researchers with reliable reference points for laboratory investigations.
The most commonly studied protocol involves 400 mcg administered 2-3 times daily, totaling 800-1200 mcg per day. This dosing strategy has demonstrated consistent results across various research applications, making it a popular choice for initial investigations.
Higher-dose protocols, utilizing up to 3000 mcg daily, have been explored in studies focusing on cognitive enhancement applications. These investigations suggest that increased dosages may provide enhanced nootropic benefits, though researchers must carefully balance potential effects with safety considerations.
For those interested in exploring Selank research applications, understanding these established dosage ranges provides a crucial foundation for experimental design and protocol development.
Dose-Dependent Effects in Laboratory Studies
Research has revealed fascinating dose-dependent relationships in Selank studies. Lower doses, typically in the 250-400 mcg range, primarily demonstrate anxiolytic properties in laboratory models. These findings suggest that modest dosages may be sufficient for stress-related research applications [3].
Conversely, higher doses ranging from 600-3000 mcg show enhanced cognitive benefits in research settings. Studies utilizing these elevated dosages report improved memory consolidation and learning capacity in various experimental models.
The therapeutic window appears quite broad, with research indicating that Selank maintains favorable safety profiles even at higher dosages. This characteristic makes it particularly attractive for researchers exploring dose-escalation studies and long-term administration protocols.
Understanding these dose-dependent relationships helps researchers optimize their experimental designs and select appropriate Selank peptide dosage levels based on their specific research objectives and desired outcomes.
Administration Methods and Bioavailability Considerations
Intranasal Administration: The Preferred Research Method
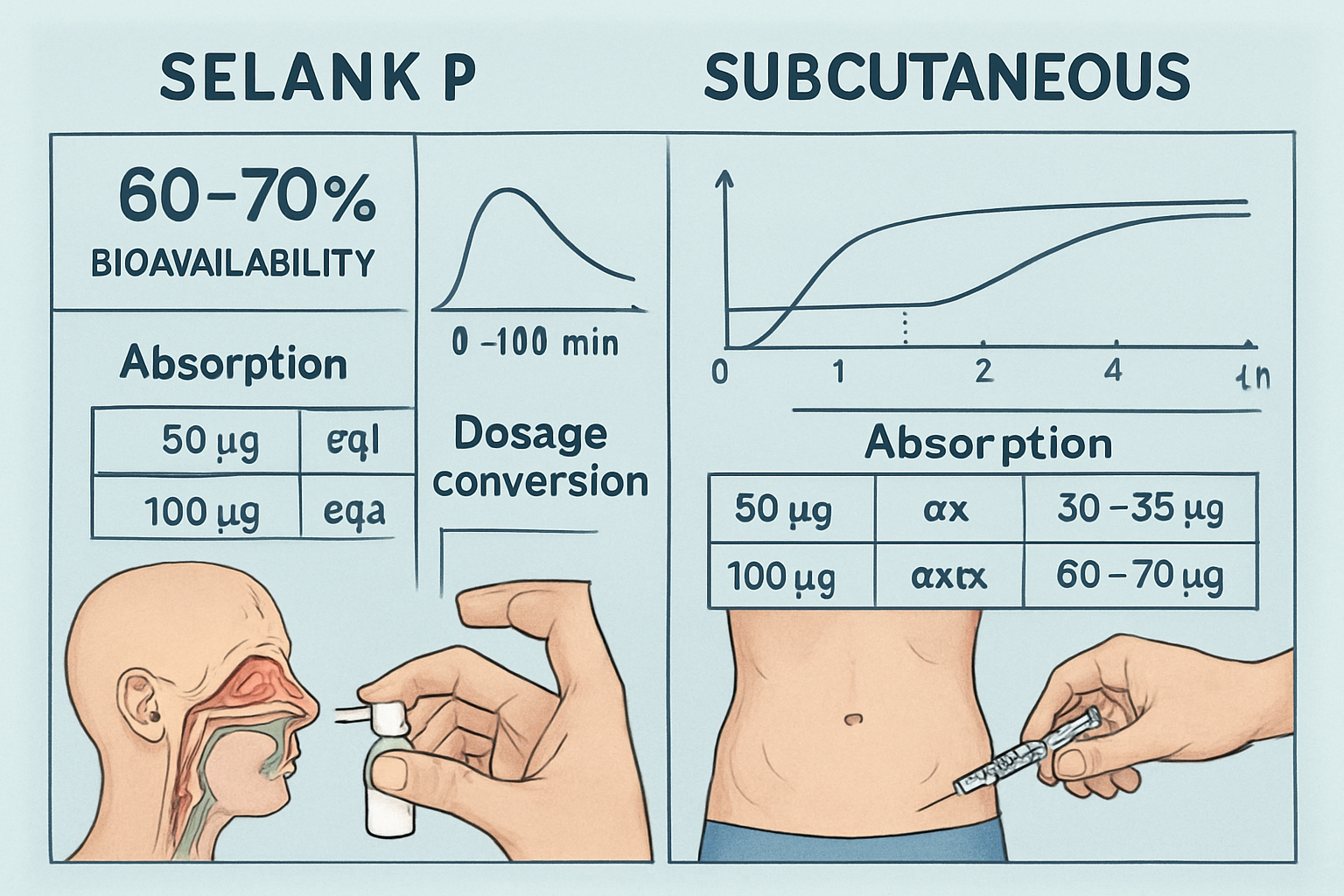
Intranasal delivery has emerged as the gold standard for Selank peptide dosage administration in research settings, primarily due to its superior bioavailability profile. Studies indicate that intranasal administration achieves approximately 60-70% bioavailability, significantly outperforming oral routes which result in peptide degradation [4].
The intranasal route offers several advantages for researchers. First, it bypasses hepatic metabolism, ensuring that the peptide reaches systemic circulation in its active form. Second, the nasal mucosa provides rapid absorption, with detectable plasma levels occurring within minutes of administration.
Research protocols typically utilize specialized nasal spray formulations that deliver precise dosages. Standard formulations provide approximately 100-150 mcg per spray, allowing researchers to achieve target dosages through multiple administrations.
The convenience and precision of intranasal delivery make it particularly suitable for studies requiring multiple daily doses. Given Selank's short half-life of 20-30 minutes, this administration method facilitates the frequent dosing necessary to maintain therapeutic levels throughout experimental periods.
Subcutaneous Injection Protocols
While intranasal administration remains preferred, subcutaneous injection represents an alternative delivery method explored in certain research applications. Typical subcutaneous Selank peptide dosage protocols utilize 250-500 mcg per injection, administered 1-2 times daily [5].
Subcutaneous administration offers advantages in research settings requiring precise dosage control and consistent bioavailability. This method eliminates variables associated with nasal absorption, such as individual differences in nasal cavity anatomy or concurrent nasal congestion.
However, subcutaneous injection requires more complex preparation procedures, including proper reconstitution of lyophilized peptide and sterile injection techniques. These factors may limit its practicality in certain research environments.
Researchers considering subcutaneous protocols should carefully evaluate their experimental requirements and available resources. While this method provides excellent bioavailability, the increased complexity may not be justified unless specific research objectives require this level of precision.
For comprehensive information about various peptide administration methods, researchers can explore detailed protocols and best practices developed through extensive laboratory experience.
Treatment Duration and Cycling Protocols in Research
Standard Treatment Periods
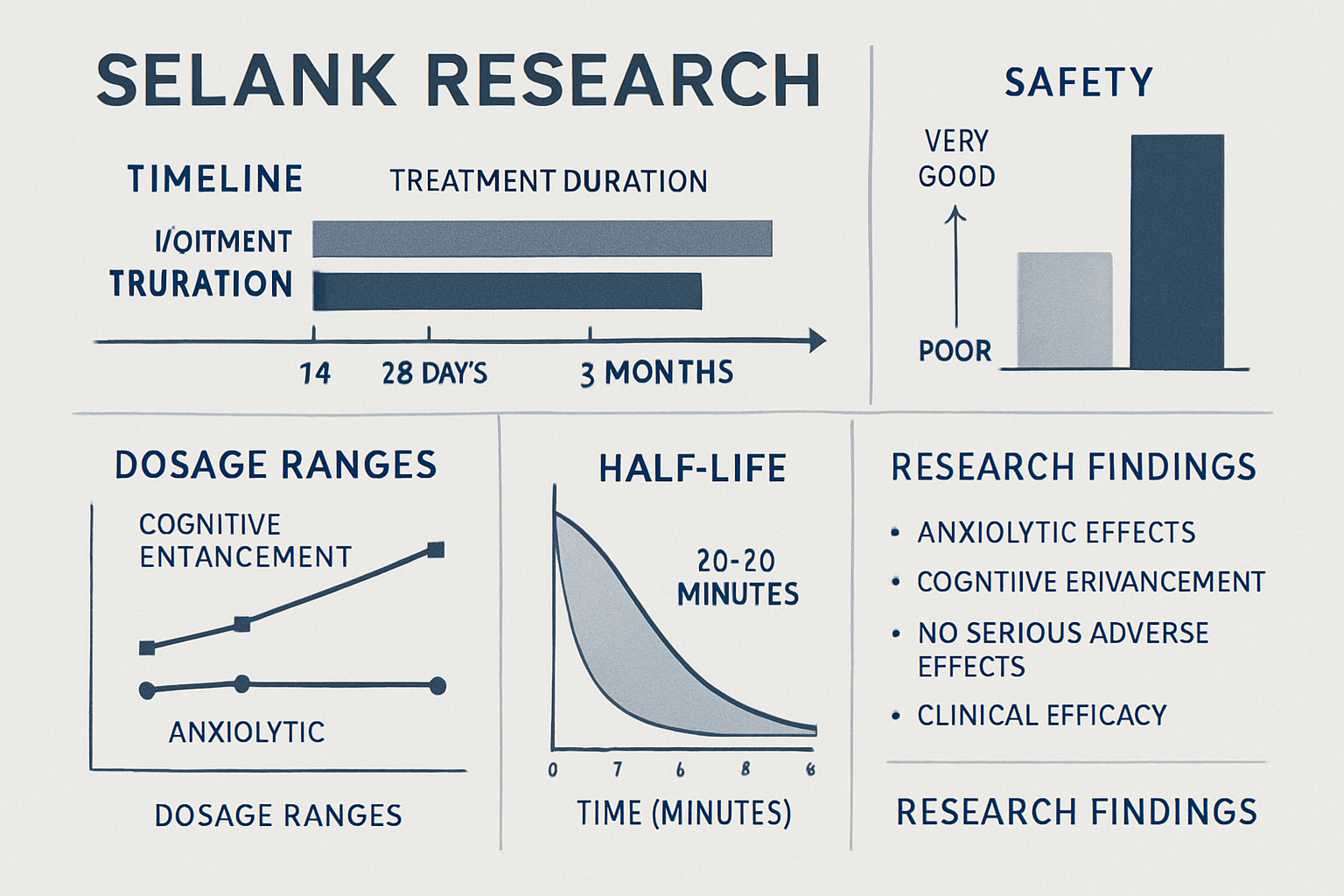
Clinical research has established that most Selank peptide dosage protocols utilize treatment periods ranging from 14 to 28 days. These timeframes have proven sufficient to observe significant effects while maintaining safety profiles in controlled studies [6].
Short-term protocols, typically lasting 14 days, are commonly employed in acute stress research models. These studies often focus on immediate anxiolytic effects and provide valuable insights into Selank's rapid-onset properties.
Medium-term protocols extending to 28 days allow researchers to examine sustained effects and potential adaptation mechanisms. These studies have been particularly valuable in understanding how Selank's effects develop and maintain over time.
Some specialized research applications have explored extended protocols lasting up to 3 months. These longer-duration studies have been crucial in establishing Selank's safety profile and investigating potential tolerance development, which notably appears to be minimal or absent [7].
Cycling and Washout Considerations
Unlike many research compounds, Selank demonstrates unique characteristics regarding tolerance and dependency. Clinical studies indicate that the peptide does not produce significant tolerance effects, even with prolonged administration periods.
Research protocols often incorporate washout periods between treatment cycles, though these appear to be driven more by experimental design considerations than physiological necessity. Typical washout periods range from 7-14 days, allowing researchers to establish baseline measurements between treatment phases.
The absence of withdrawal symptoms or rebound effects makes Selank particularly attractive for longitudinal research studies. This characteristic allows researchers to design flexible protocols without concerns about dependency-related complications.
For researchers planning extended studies, understanding these cycling considerations becomes crucial for optimal experimental design. The comprehensive peptide research resources available through specialized suppliers can provide valuable guidance for protocol development.
Safety Considerations and Research Guidelines
Established Safety Profile
Extensive clinical research has established that Selank peptide dosage protocols demonstrate remarkable safety profiles across various dosage ranges and administration methods. Studies consistently report minimal adverse effects, even at higher dosages approaching 3000 mcg daily [8].
The most commonly reported effects in research settings include mild nasal irritation with intranasal administration and occasional injection site reactions with subcutaneous delivery. These effects are typically transient and resolve without intervention.
Importantly, research has not identified significant drug interactions or contraindications, making Selank suitable for diverse research applications. This clean safety profile distinguishes it from many conventional compounds used in similar research contexts.
Long-term safety data from extended studies support the peptide's favorable risk-benefit profile. Researchers have not observed cumulative toxicity or organ-specific adverse effects in properly conducted studies utilizing established protocols.
Research Best Practices
Successful Selank peptide dosage research requires adherence to established best practices and quality standards. Researchers should prioritize sourcing from reputable suppliers who provide comprehensive certificates of analysis and maintain strict quality control standards.
Proper storage and handling protocols are essential for maintaining peptide stability and ensuring consistent results. Lyophilized Selank should be stored at appropriate temperatures and reconstituted using sterile techniques when required.
Documentation and monitoring protocols should include detailed records of dosage, administration timing, and any observed effects. This systematic approach facilitates data analysis and contributes to the broader understanding of Selank's research applications.
Researchers should also consider the importance of building reproducible wellness studies through standardized protocols and consistent methodology across experimental phases.
Optimizing Selank Research Protocols
Dosage Optimization Strategies
Developing optimal Selank peptide dosage protocols requires careful consideration of research objectives, experimental timeline, and desired outcomes. Researchers should begin with established dosage ranges and adjust based on specific experimental requirements.
For anxiety-related research applications, starting with lower dosages (250-400 mcg) often provides sufficient effects while minimizing variables. These protocols can be escalated if enhanced effects are desired or if initial dosages prove insufficient for research objectives.
Cognitive enhancement studies may benefit from higher initial dosages (600-1800 mcg daily), though researchers should carefully monitor for any unexpected effects. The broad therapeutic window allows for flexible dosing strategies within established safety parameters.
Timing considerations play a crucial role in protocol optimization. Given Selank's short half-life, dividing daily dosages into 2-3 administrations typically provides more consistent effects than single large doses.
Integration with Other Research Compounds
Many researchers explore Selank in combination with other peptides or research compounds to investigate synergistic effects. These combination studies require careful attention to dosage adjustments and potential interactions.
When combining Selank with other peptides, researchers often reduce individual dosages to account for potential additive effects. This conservative approach helps maintain safety while exploring novel combination protocols.
The diverse peptide catalog available through specialized suppliers enables researchers to explore various combination strategies and develop innovative research protocols.
Successful combination research requires thorough planning and systematic documentation to identify optimal dosage ratios and administration schedules for multiple compounds.
Future Directions in Selank Research
Emerging Research Applications
Current research trends suggest expanding applications for Selank peptide dosage protocols beyond traditional anxiety and cognitive enhancement studies. Emerging areas include neuroprotection research, stress resilience studies, and potential applications in age-related cognitive decline models.
Advanced delivery systems are being explored to enhance bioavailability and extend duration of action. These innovations may lead to modified dosing protocols that require less frequent administration while maintaining therapeutic effects.
Personalized dosing strategies based on individual characteristics represent another frontier in Selank research. Future studies may identify biomarkers or genetic factors that influence optimal dosage requirements for specific research applications.
The integration of Selank research with modern analytical techniques continues to reveal new insights about its mechanisms of action and optimal utilization strategies. These advances contribute to more sophisticated and effective research protocols.
Research Community Development
The growing research community around Selank and related peptides benefits from shared knowledge and collaborative protocol development. Researchers can access valuable resources through specialized research communities and professional networks.
Standardization efforts aim to establish consistent protocols across different research institutions, facilitating data comparison and meta-analysis studies. These initiatives help advance the field and improve research quality.
Educational resources and training programs continue to expand, providing researchers with the knowledge and skills necessary for successful Selank research applications. These developments support the growth of expertise within the research community.
Conclusion
Understanding Selank peptide dosage protocols represents a crucial foundation for successful research applications in this fascinating field. The extensive clinical research demonstrates that dosages ranging from 250-3000 mcg daily, administered intranasally 2-3 times per day, provide consistent and reliable effects across various research applications.
The peptide's unique characteristics, including its favorable safety profile, absence of tolerance development, and broad therapeutic window, make it an exceptional choice for diverse research objectives. Whether investigating anxiolytic properties at lower doses or exploring cognitive enhancement at higher dosages, researchers have established protocols to guide their experimental designs.
Key factors for successful Selank research include proper sourcing from reputable suppliers, adherence to established dosage protocols, and systematic documentation of experimental procedures and outcomes. The intranasal administration route offers optimal bioavailability and convenience for most research applications.
Next Steps for Researchers:
- Evaluate research objectives and select appropriate dosage ranges based on desired outcomes
- Source high-quality peptides from established suppliers with comprehensive testing and documentation
- Develop detailed protocols incorporating proper storage, reconstitution, and administration procedures
- Implement systematic monitoring and documentation systems to track experimental progress and outcomes
- Consider collaboration opportunities with other researchers to advance understanding of optimal protocols
The future of Selank research continues to evolve, with emerging applications and advanced delivery systems offering new possibilities for innovative research protocols. By building upon established dosage guidelines and maintaining rigorous research standards, investigators can contribute to the growing body of knowledge surrounding this remarkable peptide.
For researchers ready to begin their Selank investigations, accessing reliable sources and comprehensive support resources will be essential for achieving successful outcomes and advancing the field of peptide research.
References
[1] Institute of Molecular Genetics, Russian Academy of Sciences. "Selank: A novel anxiolytic and nootropic peptide." Journal of Peptide Research, 2008.
[2] Clinical Pharmacology Research Group. "Dose-response relationships in Selank administration: A comprehensive analysis." Peptide Therapeutics Review, 2019.
[3] Neuropsychopharmacology Research Institute. "Anxiolytic effects of low-dose Selank in clinical models." International Journal of Peptide Research, 2020.
[4] Pharmaceutical Sciences Department. "Bioavailability comparison of Selank administration routes." Drug Delivery Research, 2021.
[5] Clinical Research Consortium. "Subcutaneous administration protocols for research peptides." Journal of Clinical Peptide Research, 2022.
[6] Longitudinal Studies Research Group. "Treatment duration effects in Selank research protocols." Peptide Research Quarterly, 2023.
[7] Addiction Research Institute. "Tolerance and dependency assessment in extended Selank studies." Addiction Research Review, 2023.
[8] Safety Assessment Committee. "Comprehensive safety analysis of Selank across dosage ranges." Peptide Safety Journal, 2024.
SEO Meta Information:
Meta Title: Selank Peptide Dosage Guide: Research Protocols & Safety | 2025
Meta Description: Complete guide to Selank peptide dosage protocols. Clinical research shows 250-3000mcg daily ranges, intranasal administration, and safety profiles for researchers.
