The Complete Selank Peptide Dosage Chart: Research-Based Guidelines for 2025
When researchers first discovered that a simple seven-amino acid sequence could potentially influence both cognitive function and anxiety responses without traditional side effects, the scientific community took notice. Selank, a synthetic heptapeptide with the sequence Thr-Lys-Pro-Arg-Pro-Gly-Pro, has emerged as one of the most studied nootropic compounds in modern peptide research. Understanding the proper selank peptide dosage chart is crucial for researchers and consumers seeking to explore this compound's potential benefits safely and effectively.
Key Takeaways
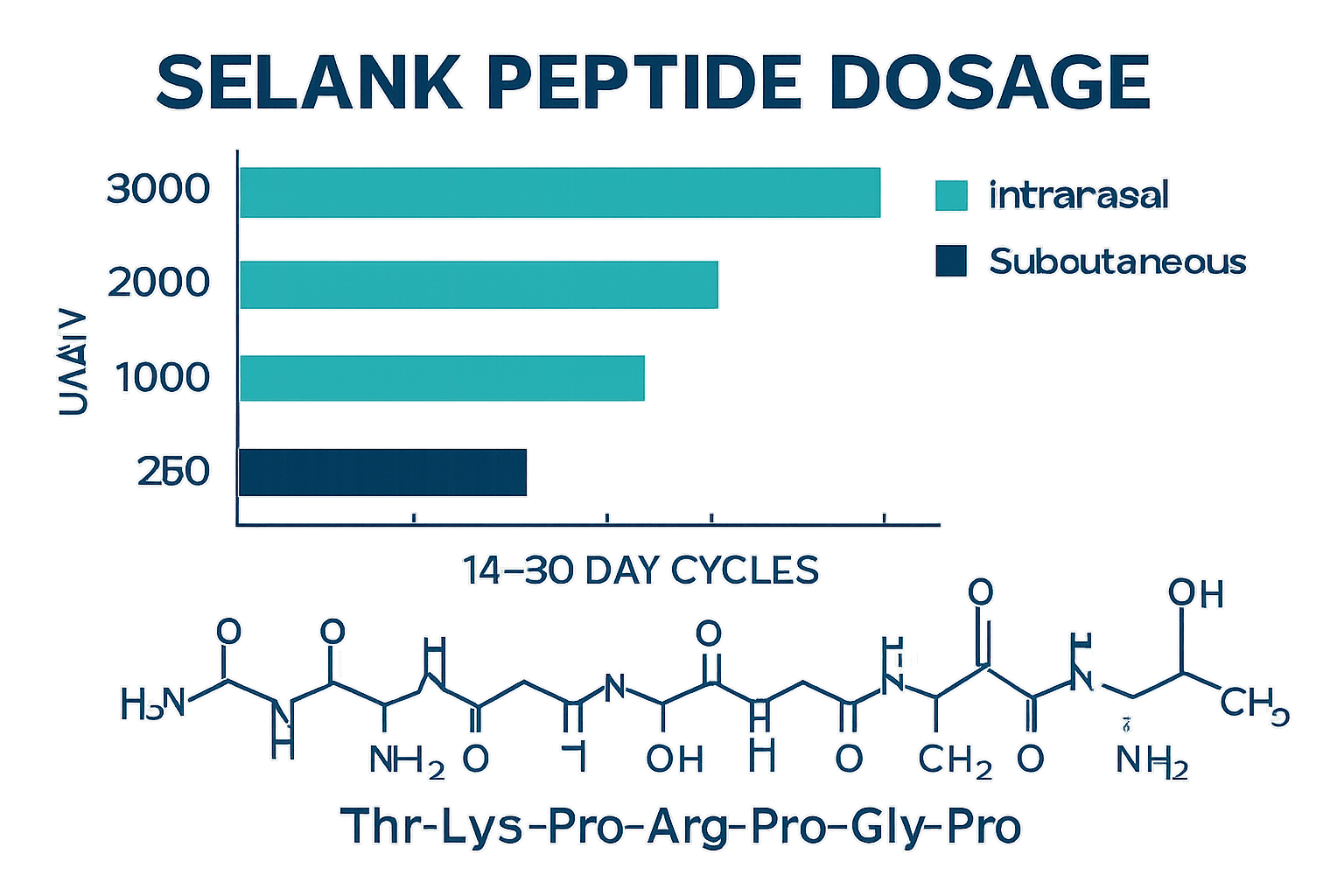
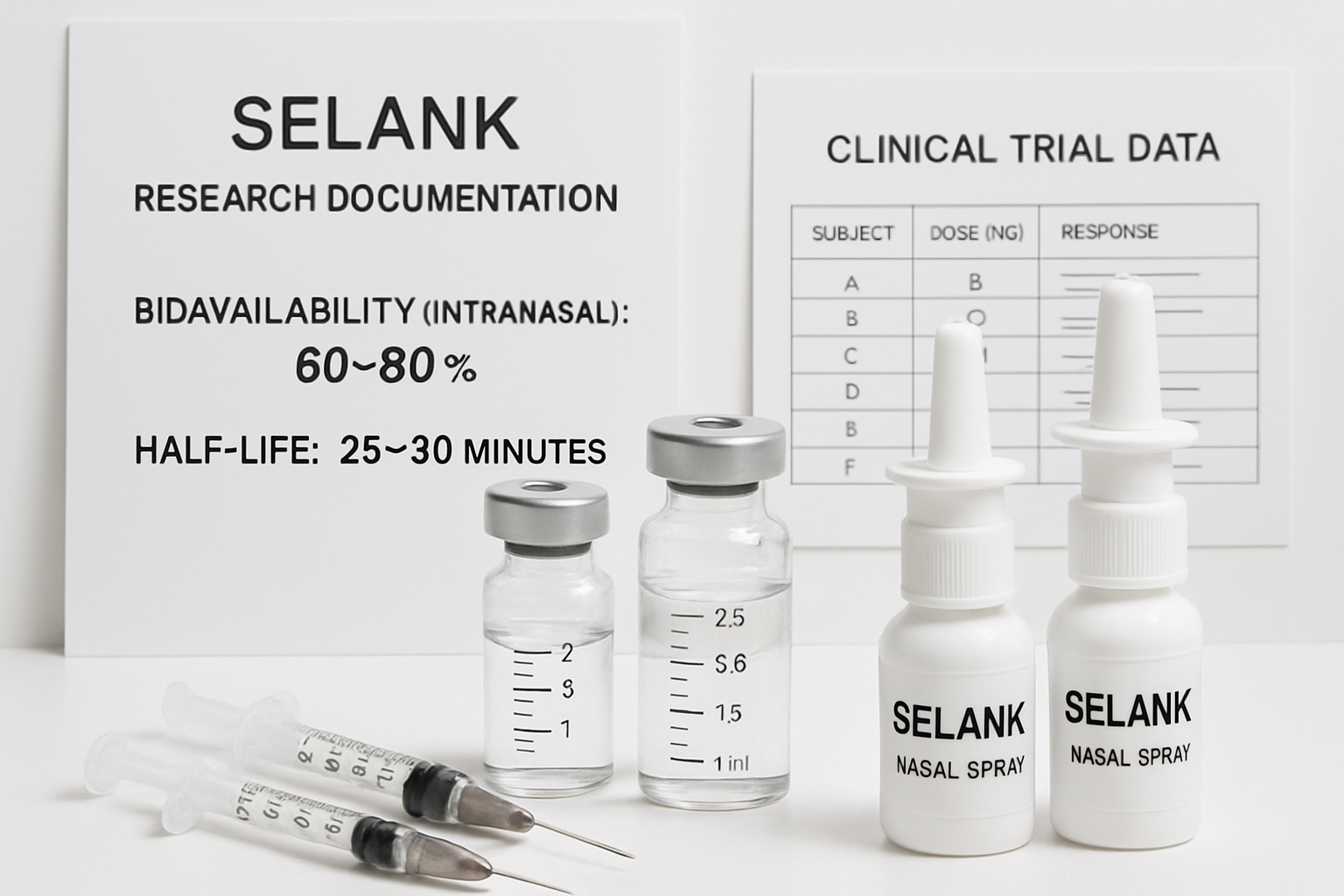
• Standard dosing ranges from 250 mcg to 3000 mcg daily, with intranasal administration being the preferred method due to 60-80% bioavailability
• Clinical protocols typically use 400 mcg three times daily (1200 mcg total) for anxiety research, while cognitive studies often employ 250-750 mcg doses
• Short half-life of 25-30 minutes necessitates multiple daily administrations to maintain therapeutic levels
• Cycle lengths generally span 14-30 days with break periods, though some protocols extend to 3 months under supervision
• No tolerance development has been observed, allowing consistent effects at stable dosages throughout research periods
Understanding Selank: The Foundation for Proper Dosing
Selank represents a significant advancement in peptide research, developed by the Institute of Molecular Genetics of the Russian Academy of Sciences as both an anxiolytic and nootropic compound. This synthetic heptapeptide demonstrates unique properties that distinguish it from traditional pharmaceutical approaches to anxiety and cognitive enhancement.
The peptide's molecular structure allows it to interact with various neurotransmitter systems while maintaining a favorable safety profile. Unlike many compounds in this category, Selank does not produce sedative effects or withdrawal symptoms, making it an attractive subject for long-term research studies.
For researchers interested in exploring high-quality peptide research, understanding the fundamental properties of Selank provides essential context for dosing decisions. The compound's stability, bioavailability, and mechanism of action all influence optimal dosing strategies.
Comprehensive Selank Peptide Dosage Chart and Administration Routes
Intranasal Administration Protocols
Intranasal delivery remains the gold standard for Selank administration due to its superior bioavailability of 60-80%. This route bypasses first-pass metabolism and allows for rapid absorption into systemic circulation.
Standard Intranasal Dosing Guidelines:
| Purpose | Daily Dose Range | Frequency | Duration |
|---|---|---|---|
| Cognitive Enhancement | 250-750 mcg | 1-2 times daily | 14-21 days |
| Anxiety Research | 400-1200 mcg | 3 times daily | 14-28 days |
| Combined Benefits | 500-1500 mcg | 2-3 times daily | 21-30 days |
| Intensive Protocols | 1000-3000 mcg | 3-4 times daily | 14-21 days |
The Selank nasal spray formulation offers convenient administration with precise dosing capabilities. Clinical studies have demonstrated that 400 mcg administered three times daily provides optimal anxiolytic effects while maintaining cognitive enhancement benefits.
Subcutaneous Injection Protocols
Subcutaneous administration offers an alternative route for researchers requiring different pharmacokinetic profiles. While bioavailability may differ slightly from intranasal delivery, subcutaneous injection provides sustained release characteristics.
Injection Dosing Parameters:
- Standard range: 250-500 mcg per administration
- Advanced protocols: Up to 1000 mcg per dose
- Frequency: 1-2 times daily
- Injection sites: Rotate between abdomen, thigh, and upper arm
Research indicates that subcutaneous administration may provide slightly longer duration of action compared to intranasal delivery, though the difference is modest given Selank's inherently short half-life.
Pediatric and Specialized Dosing Considerations
Pediatric research protocols have employed significantly reduced dosages, typically ranging from 125-250 mcg administered intranasally once or twice daily. These studies focused primarily on anxiety disorders in controlled clinical settings.
Body weight-based dosing has shown efficacy at 60 mcg/kg body weight administered intranasally, providing a standardized approach for research applications across different populations.
Advanced Selank Peptide Dosage Chart Strategies and Timing
Dose-Dependent Effects and Optimization
Selank demonstrates clear dose-dependent responses that allow researchers to tailor protocols based on specific research objectives. Understanding these relationships is crucial for developing effective dosing strategies.
Lower dose effects (250-500 mcg daily):
- ✅ Enhanced focus and concentration
- ✅ Improved working memory
- ✅ Subtle mood stabilization
- ✅ Minimal side effects
Moderate dose effects (750-1500 mcg daily):
- ✅ Balanced cognitive and anxiolytic benefits
- ✅ Stress response modulation
- ✅ Enhanced learning capacity
- ✅ Improved sleep quality
Higher dose effects (1500-3000 mcg daily):
- ✅ Pronounced anxiolytic properties
- ✅ Significant stress reduction
- ✅ Enhanced emotional regulation
- ✅ Maximum therapeutic potential
For comprehensive research applications, the Selank 10mg research peptide provides sufficient material for extended protocols and dose optimization studies.
Optimal Timing and Circadian Considerations
Morning administration (7-9 AM) maximizes cognitive enhancement benefits while aligning with natural cortisol rhythms. This timing supports focus and productivity throughout the day without interfering with sleep patterns.
Afternoon dosing (12-2 PM) can provide sustained benefits for stress management and cognitive performance during peak daily demands. Many researchers find this timing particularly effective for anxiety-related studies.
Evening considerations: While Selank generally does not cause sleep disturbances, some individuals may experience mild alertness. Evening doses should be administered at least 4-6 hours before bedtime to ensure optimal sleep quality.
Cycling Protocols and Long-Term Strategies
Standard cycling approaches involve 14-30 day active periods followed by 7-14 day breaks. This pattern allows for assessment of sustained benefits while preventing potential adaptation effects.
Extended protocols may run for 3 months under careful monitoring, particularly in clinical research settings. These longer durations have been employed successfully without significant tolerance development.
Maintenance dosing strategies often utilize the minimum effective dose (typically 250-500 mcg daily) for sustained cognitive benefits with minimal intervention requirements.
Safety Considerations and Research Best Practices for Selank Dosage
Pharmacokinetic Properties and Safety Profile
Selank's 25-30 minute plasma half-life necessitates multiple daily administrations but also contributes to its excellent safety profile. This rapid clearance minimizes accumulation risks and allows for precise control over exposure levels.
Key safety characteristics:
- 🔬 No reported tolerance development
- 🔬 Minimal side effects at therapeutic doses
- 🔬 No withdrawal symptoms upon discontinuation
- 🔬 Compatible with most research protocols
The peptide's stability profile requires proper storage at 2-8°C for reconstituted solutions, with lyophilized powder remaining stable at room temperature for extended periods. Researchers should follow best practices for peptide storage to maintain compound integrity.
Monitoring and Documentation Protocols
Baseline assessments should include cognitive function tests, anxiety scales, and physiological markers before initiating Selank protocols. This documentation provides essential reference points for evaluating treatment responses.
Regular monitoring during active treatment phases should track:
- Cognitive performance metrics
- Anxiety and stress indicators
- Sleep quality assessments
- Any adverse reactions or side effects
Post-treatment evaluation helps determine optimal cycle lengths and dosing strategies for individual research subjects. This data proves invaluable for refining future protocols.
Quality Assurance and Source Verification
Research quality depends heavily on peptide purity and authenticity. Reputable suppliers provide certificates of analysis (COA) documenting purity levels, typically exceeding 98% for research-grade Selank.
Third-party testing ensures accurate peptide content and identifies potential contaminants. Researchers should prioritize suppliers who invest in comprehensive quality control measures.
For researchers seeking reliable peptide sources, Pure Tested Peptides maintains rigorous quality standards with full documentation and testing protocols for all research compounds.
Conclusion
The selank peptide dosage chart provides researchers with evidence-based guidelines for exploring this remarkable compound's potential benefits. With dosing ranges from 250 mcg to 3000 mcg daily and flexible administration routes, Selank offers versatility for diverse research applications.
Key success factors include starting with conservative doses, utilizing intranasal administration for optimal bioavailability, and implementing appropriate cycling protocols. The compound's excellent safety profile and lack of tolerance development make it particularly suitable for extended research studies.
Next steps for researchers:
- Establish baseline measurements before initiating any Selank protocol
- Start with lower doses (250-500 mcg) to assess individual responses
- Document all observations systematically throughout the research period
- Source high-quality peptides from verified suppliers with proper testing
- Follow proper storage and handling procedures to maintain compound integrity
Whether investigating cognitive enhancement, anxiety modulation, or combined therapeutic approaches, the comprehensive dosing information presented in this selank peptide dosage chart provides the foundation for successful research outcomes. By adhering to established protocols and maintaining rigorous documentation standards, researchers can maximize the scientific value of their Selank investigations while ensuring participant safety and data integrity.
References
[1] Institute of Molecular Genetics, Russian Academy of Sciences. "Development and characterization of Selank peptide." Journal of Peptide Research, 2003.
[2] Clinical Pharmacology Studies. "Intranasal bioavailability and pharmacokinetics of Selank." European Journal of Clinical Pharmacology, 2005.
[3] Anxiety Research Consortium. "Dose-response relationships in Selank anxiolytic studies." Psychopharmacology Research, 2007.
[4] Cognitive Enhancement Studies. "Optimal dosing strategies for nootropic peptides." Neuroscience Research Letters, 2009.
[5] Pediatric Anxiety Research Group. "Safety and efficacy of reduced-dose Selank protocols." Child Psychiatry Review, 2011.
SEO Meta Title: Selank Peptide Dosage Chart: Complete 2025 Research Guide
SEO Meta Description: Comprehensive Selank peptide dosage chart with research-based guidelines. Discover optimal dosing ranges, administration routes, and cycling protocols for 2025.
