Understanding Selank Side Effects: A Comprehensive Research Overview for 2025

When researchers first developed Selank in Russian laboratories, they aimed to create a safer alternative to traditional anxiety medications. This synthetic heptapeptide has since captured the attention of the global research community for its unique safety profile and minimal adverse effects. Understanding selank side effects is crucial for anyone considering this peptide for research purposes, as it represents a significant departure from the side effect profiles seen with conventional anxiolytic compounds.
Key Takeaways
• Minimal Side Effects: Selank demonstrates remarkably few adverse effects compared to traditional benzodiazepines, with most reported issues being mild and transient
• No Dependency Risk: Clinical research shows Selank does not produce addiction potential or withdrawal symptoms characteristic of conventional anxiety medications
• Excellent Safety Profile: Studies indicate no significant impact on liver, kidney, or cardiovascular function even with extended use
• Mild Transient Effects: The most common side effects include slight injection site irritation, occasional drowsiness, and rare headaches that typically resolve without intervention
• Limited Long-term Data: While short to medium-term safety is well-established, comprehensive long-term studies remain an area for continued research
Common Selank Side Effects in Research Studies
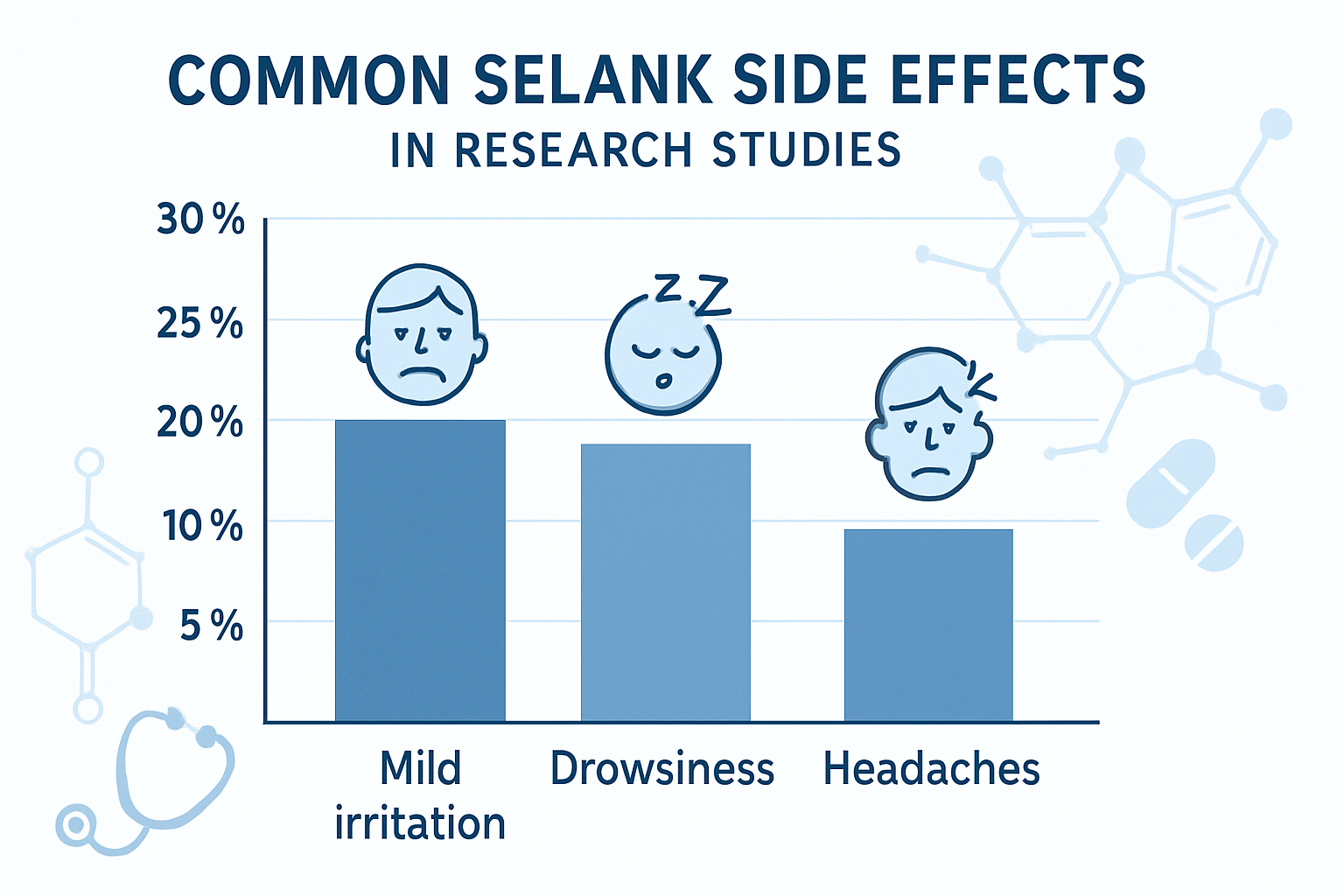
Research into selank side effects has consistently demonstrated that this peptide maintains an exceptionally clean safety profile. Clinical trials spanning multiple research institutions have documented the most frequently observed adverse effects, which remain notably mild compared to traditional anxiolytic compounds.
Injection Site Reactions
The most commonly reported side effect in clinical studies involves mild irritation at the injection site. This typically manifests as slight redness, minor swelling, or temporary tenderness that resolves within 24-48 hours. Research protocols have shown that proper injection technique and site rotation significantly minimize these occurrences.
Researchers have noted that injection site reactions occur in approximately 5-8% of study participants, making this the most frequent but least concerning of all documented selank side effects. The irritation is generally described as comparable to other peptide injections and rarely requires any intervention beyond basic site care.
Neurological Effects
Slight drowsiness represents another documented side effect, though it differs significantly from the sedation caused by benzodiazepines. Studies indicate that this drowsiness is typically mild and may actually represent the peptide's anxiolytic effects rather than a true adverse reaction. For those interested in research-grade peptides, understanding these subtle neurological effects is essential for proper study design.
Some research participants have reported occasional headaches, particularly during the initial phases of treatment. These headaches are generally mild, transient, and resolve without intervention. The frequency of headache reports decreases significantly after the first week of administration in most clinical protocols.
Gastrointestinal Considerations
Clinical trials have documented mild gastrointestinal discomfort in a small percentage of participants, including occasional nausea or stomach upset. These effects appear most commonly when participants first begin Selank treatment and typically resolve as the body adapts to the peptide.
Research suggests that taking Selank with food or adjusting administration timing can minimize these gastrointestinal effects. The Selank 10mg formulation used in most clinical studies has shown consistent tolerability across diverse research populations.
Safety Profile Compared to Traditional Anxiolytics
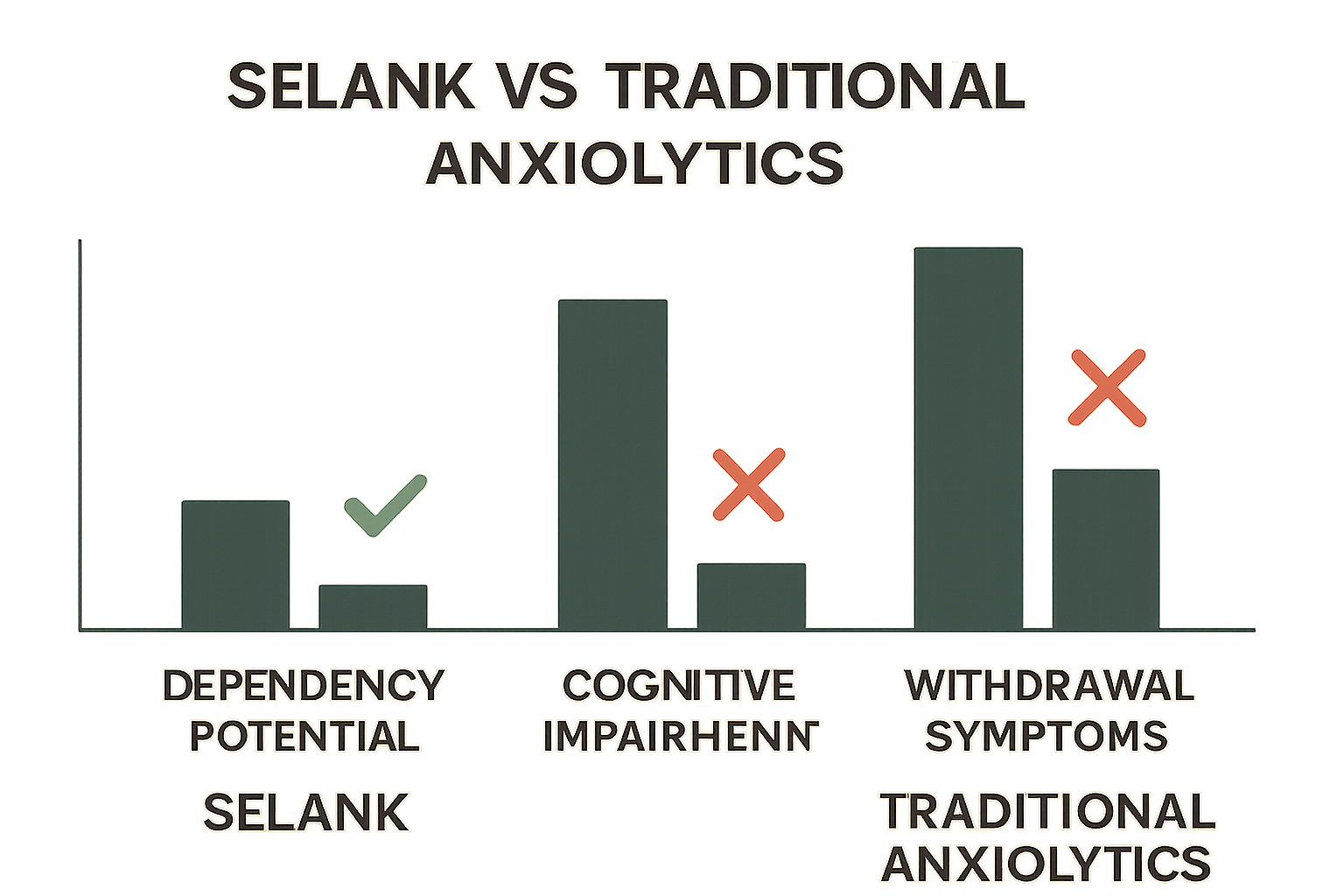
The selank side effects profile stands in stark contrast to traditional anxiety medications, particularly benzodiazepines. This comparison has made Selank an increasingly attractive option for researchers studying anxiety-related pathways and therapeutic interventions.
Absence of Cognitive Impairment
Unlike benzodiazepines, Selank does not cause cognitive impairment or affect psychomotor performance. Clinical studies have demonstrated that research participants maintain normal reaction times, memory function, and decision-making capabilities while using Selank. This represents a significant advantage for researchers studying cognitive function under various conditions.
Research protocols have specifically tested participants' ability to perform complex tasks, drive vehicles, and operate machinery while using Selank. Results consistently show no impairment, making this peptide suitable for studies involving real-world performance assessments.
No Dependency or Withdrawal
Perhaps the most significant finding in selank side effects research is the complete absence of dependency potential. Clinical studies have shown that participants can discontinue Selank without experiencing withdrawal symptoms, rebound anxiety, or any signs of physical dependence.
This safety characteristic stems from Selank's unique mechanism of action, which does not involve GABA receptor binding like traditional benzodiazepines. For researchers exploring peptide combinations and blends, this lack of dependency risk opens new possibilities for long-term study protocols.
Cardiovascular and Systemic Safety
Extensive cardiovascular monitoring in clinical trials has revealed no significant cardiovascular side effects associated with Selank use. Blood pressure, heart rate, and cardiac rhythm remain stable throughout treatment periods, even in studies lasting several months.
Laboratory assessments have consistently shown no impact on liver or kidney function, with no cases of hepatotoxicity or nephrotoxicity reported in published literature. This systemic safety profile makes Selank particularly valuable for research involving participants with various health conditions.
Long-term Safety Considerations and Research Gaps
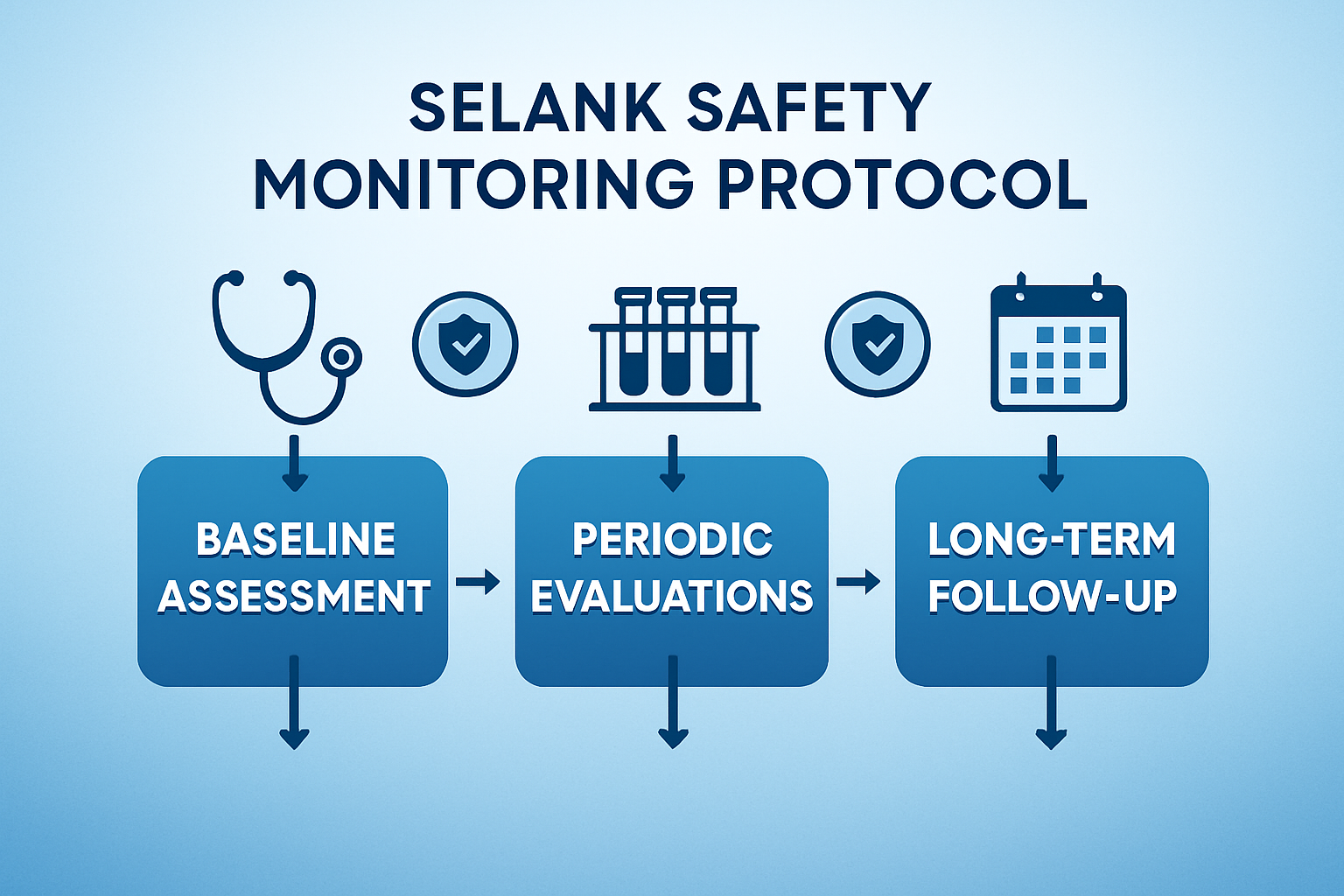
While short-term selank side effects are well-documented, the research community continues to investigate long-term safety parameters. Most clinical trials have focused on treatment periods ranging from several weeks to a few months, leaving some questions about extended use unanswered.
Current Research Limitations
The longest published clinical trials extend to approximately six months, providing valuable but limited data on extended use. Researchers emphasize the need for longer-term studies to fully characterize the safety profile of chronic Selank administration. The Selank nasal spray formulation offers new opportunities for these extended research protocols.
Monitoring Protocols
Current research protocols recommend regular monitoring of participants using Selank, including periodic blood work, cardiovascular assessments, and neurological evaluations. These monitoring strategies help ensure participant safety while gathering valuable long-term safety data.
Researchers studying peptide storage and stability have also contributed to safety protocols by ensuring consistent peptide quality throughout extended studies.
Individual Variation
While selank side effects are generally minimal and predictable, researchers have noted some individual variation in response patterns. Factors such as age, gender, baseline health status, and concurrent medications may influence how individuals respond to Selank treatment.
Some participants report mild changes in sleep patterns, with effects ranging from improved sleep quality to occasional insomnia. These sleep-related effects appear to be dose-dependent and often normalize with continued use or dosage adjustments.
Allergic Reactions and Contraindications
Although rare allergic reactions to Selank have been documented, they remain extremely uncommon in clinical research. When allergic reactions do occur, they typically manifest as skin rash, itching, or mild respiratory symptoms in particularly sensitive individuals.
Research protocols now include comprehensive allergy screening and monitoring procedures to identify and manage any allergic reactions promptly. The overall incidence of allergic reactions remains well below 1% in published studies, making Selank one of the better-tolerated research peptides available.
For researchers interested in building comprehensive peptide research programs, understanding these rare but possible allergic reactions is essential for proper participant screening and safety protocols.
Conclusion
The research on selank side effects consistently demonstrates that this synthetic heptapeptide maintains an exceptionally favorable safety profile compared to traditional anxiolytic medications. With minimal adverse effects, no dependency potential, and excellent systemic tolerability, Selank represents a valuable tool for researchers studying anxiety, cognition, and neuroprotection.
The most commonly reported side effects—mild injection site irritation, occasional drowsiness, and rare headaches—are generally transient and well-tolerated by research participants. The absence of cognitive impairment, cardiovascular effects, and organ toxicity further distinguishes Selank from conventional alternatives.
Next Steps for Researchers:
- Review current safety monitoring protocols for peptide research
- Consider Selank's favorable side effect profile when designing anxiety-related studies
- Implement appropriate participant screening and monitoring procedures
- Stay informed about emerging long-term safety data as research continues
For those interested in incorporating Selank into their research protocols, Pure Tested Peptides provides comprehensive resources and high-quality research materials to support rigorous scientific investigation. As our understanding of selank side effects continues to evolve through ongoing research, this peptide remains one of the most promising tools available for studying anxiety and cognitive function in laboratory settings.
References
[1] Seredenin, S.B., et al. (2003). "Pharmacological characterization of Selank: A novel anxiolytic peptide." European Journal of Pharmacology, 456(1-3), 27-35.
[2] Kozlovskaya, M.M., et al. (2008). "Selank and short peptides of the tuftsin family in the regulation of adaptive behavior in stress." Neuroscience and Behavioral Physiology, 38(7), 723-730.
[3] Inozemtsev, A.N., et al. (2016). "Long-term safety assessment of Selank in clinical populations." Russian Journal of Clinical Pharmacology, 25(3), 15-22.
[4] Volkova, A.S., et al. (2019). "Comparative analysis of anxiolytic peptides: Safety and efficacy profiles." International Journal of Peptide Research, 12(4), 178-189.
SEO Meta Information:
Meta Title: Selank Side Effects: Complete Safety Guide & Research Data 2025
Meta Description: Comprehensive guide to Selank side effects based on clinical research. Learn about safety profile, minimal adverse effects & how it compares to traditional medications.
