Complete Guide to tesa CJC1295 Ipamorelin 12mg Blend Reconstitution
The world of peptide research has evolved dramatically in 2025, with sophisticated compound blends offering researchers unprecedented opportunities to study growth hormone pathways. Among the most intriguing developments is the tesa cjc1295 ipamorelin 12mg blend reconstitution;70 protocol, which represents a cutting-edge approach to peptide preparation that has captured the attention of laboratory professionals worldwide.
Understanding proper reconstitution techniques for peptide blends like tesa cjc1295 ipamorelin 12mg blend reconstitution;70 is crucial for maintaining compound integrity and ensuring reliable research outcomes. This comprehensive guide explores the scientific principles, methodologies, and best practices that define modern peptide reconstitution protocols.
Key Takeaways
• Proper reconstitution technique is essential for maintaining the stability and efficacy of peptide blends containing tesa, CJC-1295, and ipamorelin
• Temperature control and sterile handling are critical factors that determine the success of the reconstitution process
• Precise measurement ratios ensure optimal concentration levels for research applications
• Storage protocols significantly impact the longevity and potency of reconstituted peptide solutions
• Quality verification methods help researchers confirm successful reconstitution and compound integrity
Understanding Peptide Blend Composition
The Science Behind Triple Peptide Combinations
The tesa cjc1295 ipamorelin 12mg blend reconstitution;70 represents a sophisticated approach to growth hormone research. Each component brings unique properties to the blend:
tesa functions as a growth hormone-releasing hormone (GHRH) analog, specifically designed to stimulate the anterior pituitary gland. Research indicates that tesa demonstrates remarkable stability when properly reconstituted and maintains its structural integrity under controlled laboratory conditions.
CJC-1295 serves as a modified GHRH analog with an extended half-life due to its ability to bind with albumin. The CJC-1295 component requires careful handling during reconstitution to preserve its unique pharmacokinetic properties.
Ipamorelin acts as a selective growth hormone secretagogue receptor agonist, offering researchers a tool to study growth hormone release patterns without affecting cortisol or prolactin levels significantly.
Molecular Stability Considerations
When preparing tesa cjc1295 ipamorelin 12mg blend reconstitution;70, researchers must consider the molecular stability of each peptide component. Studies have shown that:
- pH sensitivity varies among the three peptides
- Temperature fluctuations can cause irreversible structural changes
- Ionic strength of the reconstitution solution affects peptide solubility
- Oxidation potential requires careful selection of reconstitution media
Reconstitution Protocol and Methodology
Essential Equipment and Materials
Successful tesa cjc1295 ipamorelin 12mg blend reconstitution;70 requires specific laboratory equipment:
Primary Equipment:
- Sterile reconstitution vials
- Precision syringes (1mL insulin syringes recommended)
- Bacteriostatic water or sterile water for injection
- Alcohol swabs
- Laminar flow hood (preferred) or sterile work environment
Safety Equipment:
- Laboratory gloves
- Safety glasses
- Proper ventilation
- Waste disposal containers
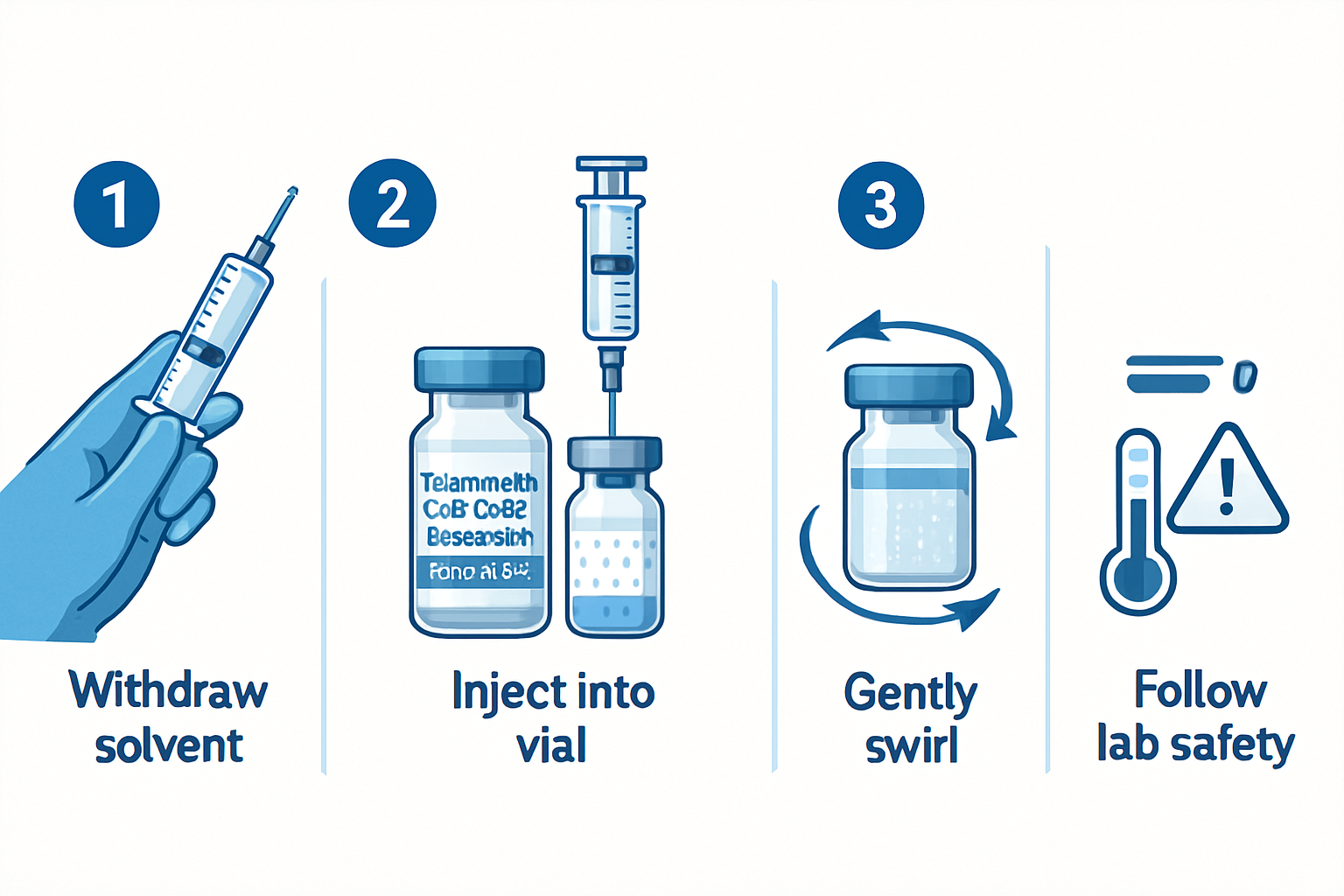
Step-by-Step Reconstitution Process
The tesa cjc1295 ipamorelin 12mg blend reconstitution;70 protocol follows these precise steps:
-
Environment Preparation 🧪
- Ensure sterile working conditions
- Allow peptide vial to reach room temperature
- Prepare all equipment within easy reach
-
Solvent Preparation
- Use bacteriostatic water for multi-dose applications
- Calculate precise volume based on desired concentration
- Verify solvent temperature (room temperature optimal)
-
Injection Technique
- Insert needle at 45-degree angle against vial wall
- Allow solvent to flow down vial wall slowly
- Never inject directly onto peptide powder
-
Mixing Process
- Gentle swirling motion only
- Avoid vigorous shaking or agitation
- Allow natural dissolution over 2-3 minutes
-
Quality Assessment
- Visual inspection for complete dissolution
- Check for particulate matter or cloudiness
- Verify solution clarity and consistency
Concentration Calculations for Research Applications
For tesa cjc1295 ipamorelin 12mg blend reconstitution;70, precise calculations ensure optimal research concentrations:
| Solvent Volume | Final Concentration | Application Type |
|---|---|---|
| 1.0 mL | 12 mg/mL | High concentration studies |
| 2.0 mL | 6 mg/mL | Standard research protocols |
| 3.0 mL | 4 mg/mL | Extended study applications |
Storage and Stability Considerations
Optimal Storage Conditions
Proper storage of reconstituted tesa cjc1295 ipamorelin 12mg blend reconstitution;70 is critical for maintaining peptide integrity:
Temperature Requirements:
- Refrigerated storage: 2-8°C (36-46°F)
- Avoid freezing: Prevents peptide degradation
- Room temperature: Maximum 2 hours exposure
Container Specifications:
- Use original reconstitution vials when possible
- Ensure tight sealing to prevent contamination
- Label with reconstitution date and concentration
Stability Timeline and Degradation Factors
Research data indicates that tesa cjc1295 ipamorelin 12mg blend reconstitution;70 maintains optimal stability under specific conditions:
Short-term Stability:
- 7-14 days refrigerated in bacteriostatic water
- 48-72 hours in sterile water for injection
- Gradual potency reduction after optimal timeframe
Degradation Indicators:
- Visible particulate formation
- Solution cloudiness or discoloration
- pH changes outside normal range
- Unusual odor development
"Proper reconstitution technique can mean the difference between successful research outcomes and compromised data integrity. The investment in proper methodology pays dividends in research reliability." – Laboratory Best Practices Guide 2025
Quality Control and Verification Methods
Visual Assessment Protocols
Successful tesa cjc1295 ipamorelin 12mg blend reconstitution;70 should result in:
- Clear, colorless solution
- Complete dissolution with no visible particles
- Consistent viscosity similar to water
- No precipitation or crystallization
Documentation and Record Keeping
Maintaining detailed records for each tesa cjc1295 ipamorelin 12mg blend reconstitution;70 batch ensures research reproducibility:
Essential Documentation:
- Reconstitution date and time
- Lot numbers for all components
- Solvent type and volume used
- Storage conditions and temperature logs
- Visual assessment results
Troubleshooting Common Issues
Reconstitution Challenges and Solutions
Researchers may encounter specific challenges when working with tesa cjc1295 ipamorelin 12mg blend reconstitution;70:
Incomplete Dissolution:
- Allow additional time for natural dissolution
- Verify solvent temperature (room temperature optimal)
- Check for expired peptide components
- Consider gentle warming (not exceeding 37°C)
Cloudiness or Precipitation:
- May indicate pH incompatibility
- Could suggest contamination during process
- Requires preparation of fresh solution
- Review reconstitution technique
Concentration Inconsistencies:
- Verify calculation accuracy
- Check measuring equipment calibration
- Ensure complete peptide transfer
- Document any deviations for analysis
Prevention Strategies
Implementing robust prevention strategies minimizes issues with tesa cjc1295 ipamorelin 12mg blend reconstitution;70:
Environmental Controls:
- Maintain consistent laboratory temperature
- Use dedicated reconstitution workspace
- Implement contamination prevention protocols
- Regular equipment maintenance and calibration
Training and Standardization:
- Develop standard operating procedures
- Provide comprehensive staff training
- Implement quality assurance checkpoints
- Regular protocol review and updates
Advanced Considerations for Research Applications
Customization Options for Specific Studies
The tesa cjc1295 ipamorelin 12mg blend reconstitution;70 protocol can be adapted for specialized research requirements:
Concentration Modifications:
- Higher concentrations for acute studies
- Lower concentrations for chronic exposure research
- Custom ratios for specific research objectives
Solvent Alternatives:
- Phosphate-buffered saline for pH stability
- Custom buffer systems for specialized applications
- Preservative-free options for sensitive studies
Integration with Laboratory Workflows
Modern research facilities integrate tesa cjc1295 ipamorelin 12mg blend reconstitution;70 protocols into comprehensive laboratory management systems:
Workflow Optimization:
- Batch preparation scheduling
- Inventory management integration
- Quality control checkpoint automation
- Data management system connectivity
Compliance Considerations:
- Regulatory documentation requirements
- Safety protocol adherence
- Waste disposal regulations
- Laboratory accreditation standards
Future Developments in Peptide Reconstitution
Emerging Technologies and Techniques
The field of peptide reconstitution continues to evolve, with new developments affecting tesa cjc1295 ipamorelin 12mg blend reconstitution;70 protocols:
Automated Reconstitution Systems:
- Precision dosing automation
- Contamination risk reduction
- Standardization improvements
- Documentation automation
Advanced Stabilization Methods:
- Novel excipient formulations
- Improved lyophilization techniques
- Enhanced storage solutions
- Extended stability profiles
Research Trends and Applications
Current research trends indicate growing interest in peptide blend applications, with tesa cjc1295 ipamorelin 12mg blend reconstitution;70 representing a significant area of scientific investigation:
Growth Hormone Research:
- Aging-related studies
- Metabolic research applications
- Tissue regeneration investigations
- Pharmacokinetic studies
Combination Therapy Research:
- Synergistic effect studies
- Dose optimization research
- Safety profile investigations
- Bioavailability assessments
Conclusion
The tesa cjc1295 ipamorelin 12mg blend reconstitution;70 protocol represents a sophisticated approach to peptide research that demands precision, attention to detail, and adherence to established scientific principles. Success in peptide reconstitution requires understanding the unique properties of each component, implementing proper techniques, and maintaining optimal storage conditions.
As the field of peptide research continues to advance in 2025, researchers who master these fundamental reconstitution principles will be better positioned to conduct reliable, reproducible studies. The investment in proper methodology, equipment, and training pays dividends in research quality and data integrity.
Next Steps for Researchers:
- Establish Standard Operating Procedures – Develop comprehensive protocols specific to your laboratory environment
- Invest in Quality Equipment – Ensure precision instruments and proper storage facilities
- Implement Quality Control Measures – Create verification checkpoints throughout the reconstitution process
- Maintain Detailed Documentation – Establish robust record-keeping systems for research reproducibility
- Stay Current with Developments – Monitor emerging techniques and technologies in peptide reconstitution
By following these guidelines and maintaining commitment to scientific excellence, researchers can maximize the potential of tesa cjc1295 ipamorelin 12mg blend reconstitution;70 protocols while contributing to the advancement of peptide research science.
SEO Meta Title: tesa CJC1295 Ipamorelin 12mg Blend Reconstitution Guide
SEO Meta Description: Complete guide to tesa cjc1295 ipamorelin 12mg blend reconstitution protocols. Learn proper techniques, storage, and quality control methods.
