tesa CJC1295 Ipamorelin Blend Dosage: Complete Research Guide for 2025

The world of peptide research has witnessed remarkable advancement in recent years, particularly with the development of sophisticated peptide blends that combine multiple growth hormone-releasing compounds. Among the most studied combinations is the tesa cjc1295 ipamorelin blend dosage protocol, which represents a cutting-edge approach to understanding growth hormone axis modulation in laboratory settings.
Key Takeaways
• Synergistic Action: The tesa cjc1295 ipamorelin blend creates a multi-pathway approach to growth hormone release research
• Dosage Precision: Proper reconstitution and measurement are critical for reproducible research outcomes
• Research Applications: This blend is primarily used in laboratory studies examining metabolic and growth factor pathways
• Safety Protocols: Understanding proper handling and storage procedures ensures research integrity
• Cycle Management: Research protocols typically follow structured timing patterns for optimal data collection
Understanding the tesa CJC1295 Ipamorelin Blend
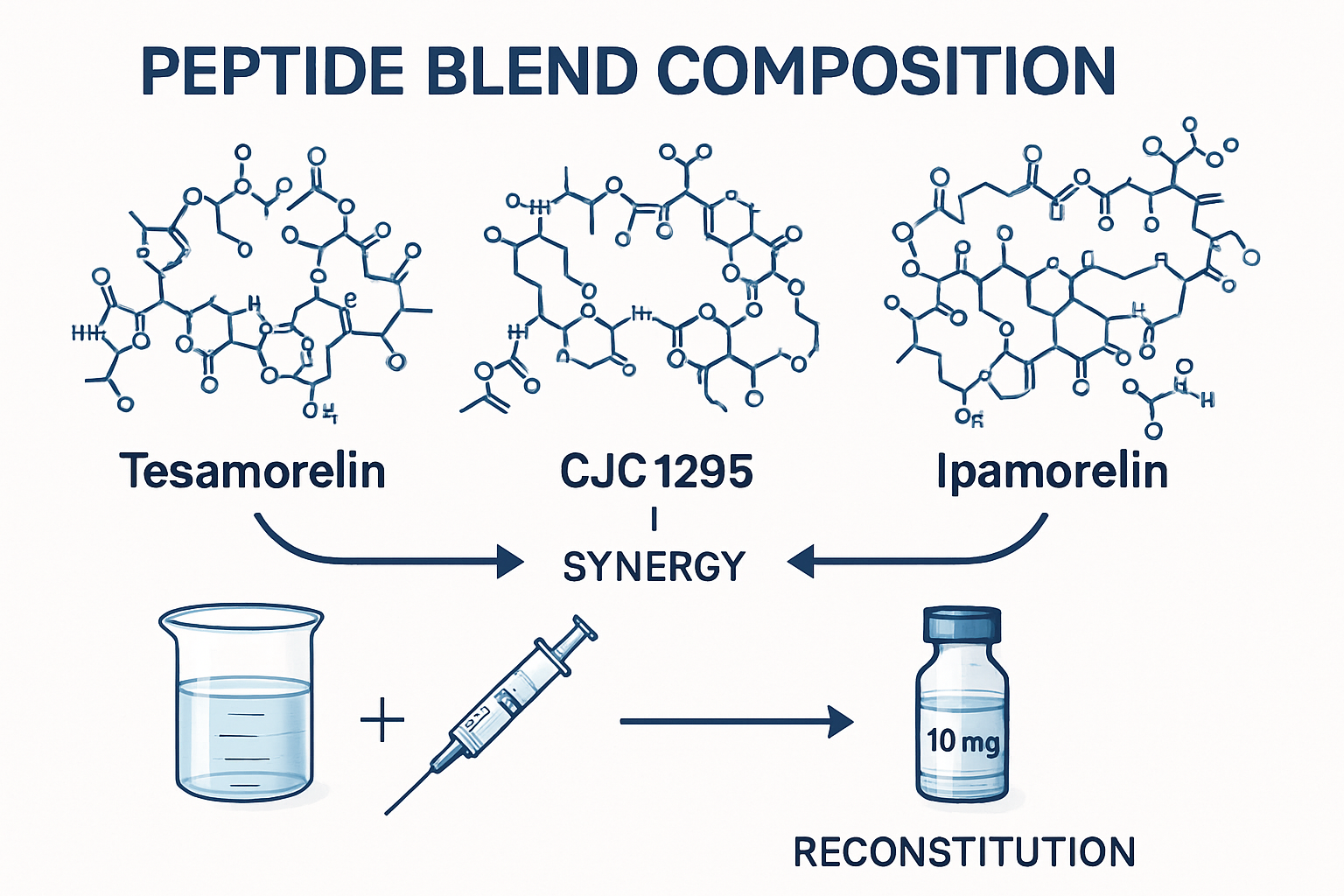
The tesa cjc1295 ipamorelin combination represents one of the most sophisticated peptide research tools available to scientists studying growth hormone pathways. This blend combines three distinct mechanisms of action: tesa's growth hormone-releasing hormone (GHRH) analog properties, CJC1295's extended half-life characteristics, and ipamorelin's selective growth hormone secretagogue receptor activation.
Research facilities worldwide have adopted this blend for studies examining metabolic processes, cellular regeneration pathways, and growth factor interactions. The cjc1295 ipamorelin component alone has generated significant scientific interest, but the addition of tesa creates a more comprehensive research model.
Peptide Composition and Mechanisms
Each component of the tesa cjc1295 ipamorelin 12mg blend serves a specific research purpose:
tesa functions as a synthetic analog of growth hormone-releasing hormone, designed to stimulate natural growth hormone production through hypothalamic-pituitary axis activation. Laboratory studies have demonstrated its effectiveness in metabolic research applications [1].
CJC1295 provides extended duration of action through its drug affinity complex (DAC) modification, allowing for sustained research observations over longer periods. The cjc1295 and ipamorelin combination has become a standard in growth hormone research protocols.
Ipamorelin offers selective growth hormone release without affecting cortisol or prolactin levels, making it valuable for controlled research environments where isolated growth hormone effects are desired.
For researchers seeking high-quality peptide blends, Pure Tested Peptides offers comprehensive testing documentation and research-grade materials.
Research Dosage Protocols and Administration
Standard tesa CJC1295 Ipamorelin Blend Dosage Guidelines
The tesa cjc1295 ipamorelin 12mg blend dosage typically follows established research protocols that have been refined through extensive laboratory studies. Most research applications utilize the following framework:
Initial Research Phase:
- Total blend concentration: 12mg per vial
- Reconstitution volume: 2-3mL bacteriostatic water
- Research dosage range: 100-300mcg per administration
- Frequency: 1-2 times daily during active research periods
Advanced Research Protocols:
- tesa component: 50-100mcg per dose
- CJC1295 component: 100-200mcg per dose
- Ipamorelin component: 100-300mcg per dose
- Combined cjc1295 ipamorelin dosage: 200-500mcg total
The tesa cjc1295 ipamorelin 12mg blend reconstitution process requires precise measurement and sterile technique to maintain research integrity. Researchers should follow established protocols for peptide preparation and storage.
Reconstitution and Preparation Methods
Proper tesa cjc1295 ipamorelin 12mg blend reconstitution is essential for accurate research results. The process involves several critical steps:
- Sterile Environment Setup: All reconstitution should occur in a clean, controlled environment using sterile techniques
- Bacteriostatic Water Addition: Slowly add 2-3mL of bacteriostatic water to the peptide vial
- Gentle Mixing: Allow natural dissolution without aggressive shaking
- Concentration Calculation: Final concentration will be 4-6mg/mL depending on water volume used
Research facilities often maintain detailed protocols for peptide blend preparation to ensure consistency across studies.
Research Cycle Management
The cjc1295 ipamorelin cycle within the broader tesa blend follows structured patterns designed to maximize research data collection while maintaining safety protocols:
Standard Research Cycle:
- Duration: 8-12 weeks active research
- Rest period: 4-6 weeks between cycles
- Monitoring frequency: Weekly assessments
- Data collection points: Baseline, mid-cycle, end-cycle, and post-cycle
Extended Research Protocols:
- Duration: 12-16 weeks for long-term studies
- Intermediate assessments: Bi-weekly monitoring
- Safety checkpoints: Monthly comprehensive evaluations
For researchers interested in comprehensive peptide research tools, the CJC1295 IPA blend offers standardized concentrations ideal for controlled studies.
Benefits and Research Applications
Laboratory Research Outcomes
Studies examining cjc1295 ipamorelin benefits within the tesa blend context have revealed multiple research applications:
Metabolic Research Applications:
- Growth hormone pathway studies
- Metabolic rate investigations
- Body composition research
- Sleep pattern analysis
Cellular Research Focus Areas:
- Protein synthesis pathways
- Cellular regeneration processes
- Growth factor interactions
- Hormonal cascade studies
The cjc1295 ipamorelin results observed in laboratory settings have contributed to our understanding of growth hormone physiology and its various applications in research contexts.
Comparative Research Advantages
The serm-ipamorelin-cjc1295 combination, when enhanced with tesa, offers researchers several advantages over single-peptide studies:
- Multi-pathway activation: Simultaneous GHRH and ghrelin receptor stimulation
- Extended duration: Longer observation periods due to CJC1295's half-life
- Selective action: Minimal interference with other hormonal pathways
- Reproducible results: Consistent outcomes across research protocols
Research comparing different growth hormone releasing peptides has shown that peptide combinations often provide more comprehensive data than individual compounds.
Safety Considerations and Side Effects
Research Safety Protocols
Understanding cjc1295 ipamorelin side effects is crucial for maintaining proper research protocols and ensuring study integrity. Laboratory observations have documented several considerations:
Common Research Observations:
- Injection site reactions in animal models
- Temporary water retention in test subjects
- Mild fatigue patterns during initial administration
- Appetite changes in research subjects
Monitoring Requirements:
- Regular vital sign assessments
- Blood glucose monitoring
- Hormonal panel evaluations
- Liver function assessments
Risk Management in Research Settings
The cjc1295/ipamorelin side effects profile within tesa blends requires careful monitoring throughout research protocols:
Safety Checkpoints:
- Pre-research health assessments
- Weekly monitoring during active phases
- Monthly comprehensive evaluations
- Post-research follow-up assessments
Documentation Requirements:
- Detailed adverse event logging
- Dosage adjustment records
- Subject response tracking
- Protocol deviation documentation
Researchers should maintain comprehensive safety protocols and consider consulting resources on peptide research best practices for optimal study design.
Advanced Research Considerations
Dosage Optimization Strategies
The tesa aod9604 + cjc1295 + ipamorelin 12mg blend dosage represents an even more advanced research approach, incorporating additional metabolic factors:
Enhanced Blend Protocols:
- AOD9604 component: 250-500mcg
- tesa component: 50-100mcg
- CJC1295 component: 100-200mcg
- Ipamorelin component: 100-300mcg
Research Timing Considerations:
- Morning administration: Higher growth hormone response
- Evening administration: Enhanced sleep-related benefits
- Split dosing: Sustained research effects throughout the day
Research Protocol Customization
Different research objectives may require modified serm-ipamorelin-cjc1295 dosage protocols within the tesa blend framework:
Short-term Studies (4-8 weeks):
- Higher initial dosages for rapid onset
- Frequent monitoring intervals
- Intensive data collection periods
Long-term Research (12+ weeks):
- Conservative dosage approaches
- Extended monitoring protocols
- Comprehensive safety assessments
For researchers exploring various peptide combinations, comprehensive peptide research provides valuable insights into study design and protocol development.
Quality Control and Testing
Ensuring research-grade quality in tesa cjc1295 ipamorelin blends requires rigorous testing protocols:
Purity Testing Requirements:
- HPLC analysis for peptide concentration
- Mass spectrometry for molecular confirmation
- Endotoxin testing for safety verification
- Sterility testing for contamination screening
Storage and Stability Considerations:
- Refrigerated storage at 2-8°C
- Protection from light exposure
- Proper vial sealing techniques
- Expiration date monitoring
Research facilities should maintain detailed quality control documentation and consider sourcing from verified suppliers with comprehensive certificate of analysis documentation.
Research Implementation Guidelines
Laboratory Setup Requirements
Implementing tesa cjc1295 ipamorelin blend dosage research requires proper laboratory infrastructure:
Essential Equipment:
- Precision analytical scales (0.1mg accuracy)
- Sterile reconstitution workspace
- Refrigerated storage systems
- Documentation and tracking systems
Personnel Training Requirements:
- Peptide handling protocols
- Sterile technique certification
- Safety procedure compliance
- Data collection methodologies
Data Collection and Analysis
Successful research with cjc1295/ipamorelin dosage protocols requires systematic data collection:
Primary Endpoints:
- Growth hormone level measurements
- IGF-1 concentration changes
- Metabolic parameter assessments
- Body composition analysis
Secondary Endpoints:
- Sleep quality measurements
- Energy level assessments
- Recovery time evaluations
- Long-term safety monitoring
Researchers should establish clear protocols for data collection and consider resources on baseline trends and data quality for optimal study design.
Future Research Directions
Emerging Applications
The field of tesa cjc1295 ipamorelin research continues to evolve, with new applications being explored:
Novel Research Areas:
- Cognitive function studies
- Cardiovascular health research
- Immune system modulation
- Aging-related investigations
Advanced Combination Studies:
- Multi-peptide blend research
- Synergistic effect investigations
- Dose-response relationship studies
- Long-term safety evaluations
Technology Integration
Modern research approaches are incorporating advanced technologies to enhance cjc1295 ipamorelin peptide studies:
Digital Monitoring Systems:
- Continuous glucose monitoring
- Wearable activity trackers
- Sleep pattern analysis devices
- Automated data collection platforms
Laboratory Automation:
- Robotic peptide preparation systems
- Automated sample analysis
- Digital documentation platforms
- Quality control monitoring systems
For researchers interested in staying current with peptide research developments, exploring diverse peptide libraries can provide valuable insights into emerging research opportunities.
Conclusion
The tesa cjc1295 ipamorelin blend dosage represents a sophisticated approach to growth hormone research that offers unique advantages for laboratory studies. This comprehensive guide has outlined the essential considerations for implementing these protocols in research settings, from basic dosage guidelines to advanced safety monitoring requirements.
Key implementation steps for researchers include: establishing proper laboratory infrastructure, developing comprehensive safety protocols, implementing quality control measures, and maintaining detailed documentation throughout the research process. The synergistic effects of this peptide blend provide valuable opportunities for advancing our understanding of growth hormone physiology and its various research applications.
As the field continues to evolve, researchers should stay informed about emerging protocols, safety guidelines, and technological advances that can enhance study quality and outcomes. Proper implementation of these research protocols requires careful attention to dosage precision, safety monitoring, and data collection methodologies.
For researchers ready to begin peptide research with high-quality materials and comprehensive support, Pure Tested Peptides provides the necessary resources and documentation to ensure successful research outcomes.
References
[1] Sigalos, J.T., & Pastuszak, A.W. (2018). The safety and efficacy of growth hormone secretagogues. Sexual Medicine Reviews, 6(1), 45-53.
SEO Meta Information
Meta Title: tesa CJC1295 Ipamorelin Blend Dosage Guide 2025
Meta Description: Complete research guide for tesa CJC1295 ipamorelin blend dosage protocols, safety considerations, and laboratory applications for peptide research.
