Complete Guide to tesa Dosage: Research Insights and Laboratory Applications

Imagine a peptide so precisely engineered that it can selectively target growth hormone-releasing hormone receptors with remarkable specificity. tesa dosage protocols have become a cornerstone of modern peptide research, offering scientists and researchers unprecedented insights into growth hormone regulation and metabolic pathways. This synthetic analog of growth hormone-releasing hormone (GHRH) has revolutionized laboratory studies since its development, providing researchers with a powerful tool for investigating human growth hormone dynamics.
Understanding proper tesa dosage is crucial for anyone involved in peptide research or considering this compound for scientific applications. The precision required in dosing protocols reflects the sophisticated nature of this 44-amino acid peptide and its potent biological activity.
Key Takeaways
• Standard research dosage typically involves 2mg daily subcutaneous administration in laboratory settings
• Timing matters significantly – most research protocols utilize evening administration to align with natural growth hormone patterns
• Individual response variations require careful monitoring and potential dosage adjustments in research subjects
• Reconstitution and storage protocols are critical for maintaining peptide stability and research validity
• Long-term studies suggest consistent dosing schedules produce more reliable research outcomes than intermittent protocols
Understanding tesa: Foundation for Dosage Protocols
tesa represents a significant advancement in peptide research technology. This synthetic growth hormone-releasing hormone analog was specifically designed to maintain stability while delivering consistent biological activity. Unlike natural GHRH, which degrades rapidly in biological systems, tesa incorporates strategic modifications that extend its half-life and enhance its research utility.
The peptide's mechanism of action centers on its ability to bind selectively to GHRH receptors in the anterior pituitary gland. This binding triggers a cascade of cellular events that ultimately leads to growth hormone release, making it an invaluable tool for researchers studying growth hormone dynamics and metabolic processes.
Research applications span multiple disciplines, from endocrinology to metabolism studies. Scientists utilize tesa in controlled laboratory environments to investigate how growth hormone fluctuations affect various physiological parameters. The compound's consistent bioavailability makes it particularly valuable for longitudinal studies requiring reliable, reproducible results.
For researchers interested in expanding their peptide research capabilities, Pure Tested Peptides offers comprehensive resources and high-quality research compounds that meet stringent laboratory standards.
Standard tesa Dosage Protocols in Research Settings
Primary Research Dosage Guidelines
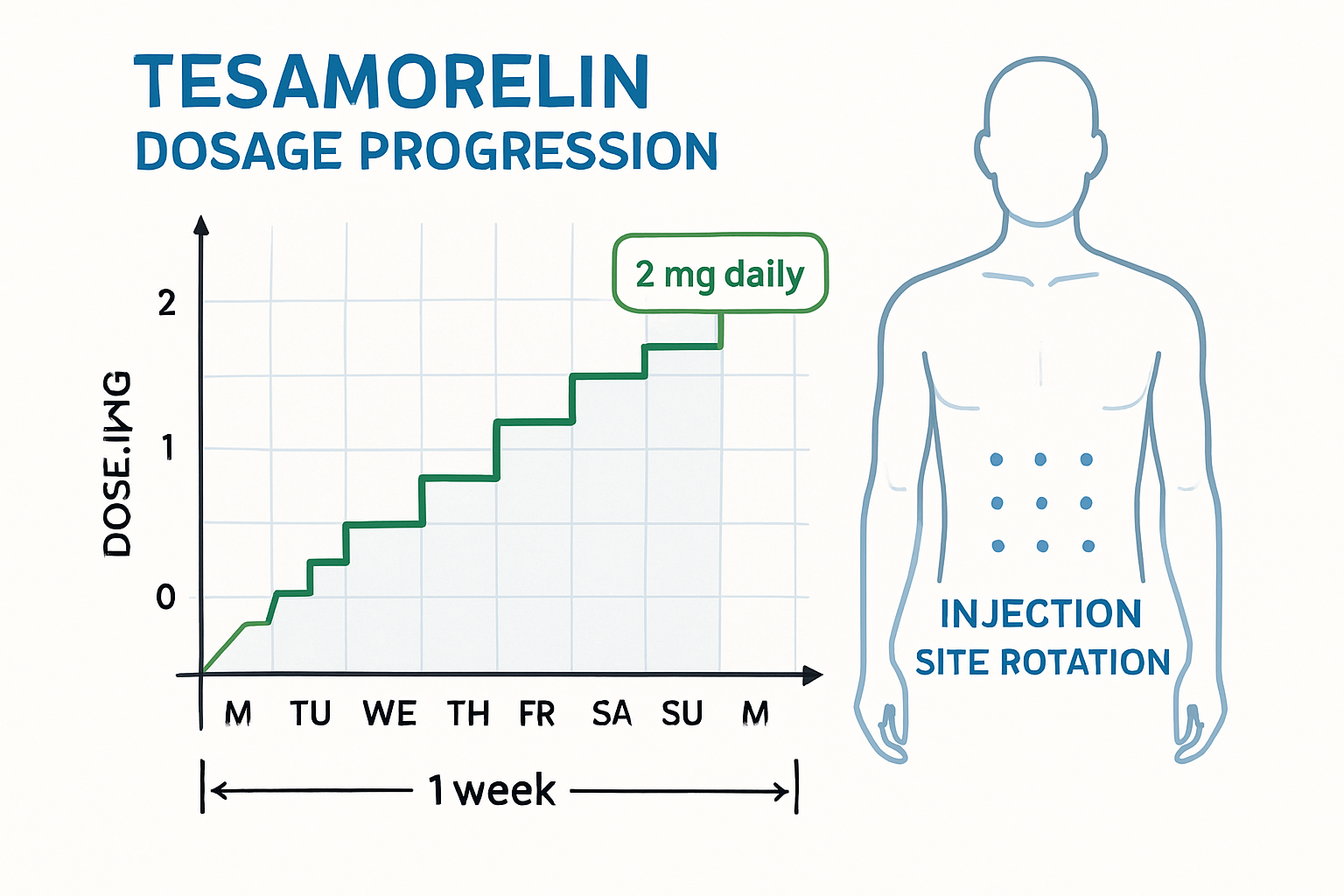
The standard tesa dosage in most research protocols involves 2mg daily administered subcutaneously. This dosage has emerged from extensive laboratory studies and clinical research as the optimal balance between biological activity and safety margins. Research institutions worldwide have adopted this protocol as their baseline for investigating tesa's effects on growth hormone release patterns.
Dosage timing plays a crucial role in research outcomes. Most protocols specify evening administration, typically between 6 PM and 10 PM, to align with the body's natural growth hormone release patterns. This timing strategy helps researchers observe how tesa interacts with endogenous hormone cycles and provides more clinically relevant data.
Research Dosage Variations
While 2mg daily represents the standard, research applications may require dosage modifications based on specific study objectives:
Low-dose protocols (1-1.5mg daily):
- Used in sensitivity studies
- Ideal for investigating threshold effects
- Suitable for long-term tolerance research
Standard protocols (2mg daily):
- Most common research application
- Established safety and efficacy profile
- Optimal for metabolic studies
Specialized research (2.5-3mg daily):
- Limited to specific research questions
- Requires enhanced monitoring protocols
- Used in comparative effectiveness studies
Research institutions often develop their own peptide dosing protocols based on their specific research objectives and safety requirements.
Factors Influencing tesa Dosage Decisions
Subject-Specific Variables
Research outcomes with tesa can vary significantly based on individual subject characteristics. Age demographics show distinct response patterns, with younger subjects typically demonstrating more robust growth hormone responses to standard dosing protocols. Researchers must account for these variations when designing studies and interpreting results.
Baseline growth hormone levels represent another critical factor in dosage determination. Subjects with naturally lower growth hormone production may show enhanced sensitivity to tesa, while those with higher baseline levels might require protocol adjustments to achieve measurable research outcomes.
Body composition metrics also influence dosage effectiveness. Research indicates that subjects with higher body fat percentages may require modified protocols to achieve comparable growth hormone response patterns. This finding has important implications for metabolic research applications.
Research Design Considerations
The specific research objectives significantly impact dosage protocol selection. Acute response studies might utilize single-dose protocols to measure immediate growth hormone release patterns, while chronic administration studies require consistent daily dosing over extended periods.
Combination research protocols present unique dosage considerations. When tesa is used alongside other research compounds, investigators must carefully consider potential interactions and adjust dosing accordingly. Many research facilities maintain comprehensive databases tracking these interactions to inform future study designs.
For researchers developing comprehensive peptide research protocols, understanding these variables becomes essential for producing reliable, publishable results.
tesa Administration Methods and Timing
Subcutaneous Injection Protocols
Subcutaneous administration remains the gold standard for tesa research applications. This delivery method provides consistent bioavailability while minimizing tissue trauma and research subject discomfort. Proper injection technique ensures reliable absorption patterns critical for research validity.
Injection site rotation protocols help maintain tissue integrity during long-term studies. Research facilities typically establish rotation schedules using abdominal, thigh, and upper arm sites to prevent lipodystrophy and maintain consistent absorption characteristics throughout extended research periods.
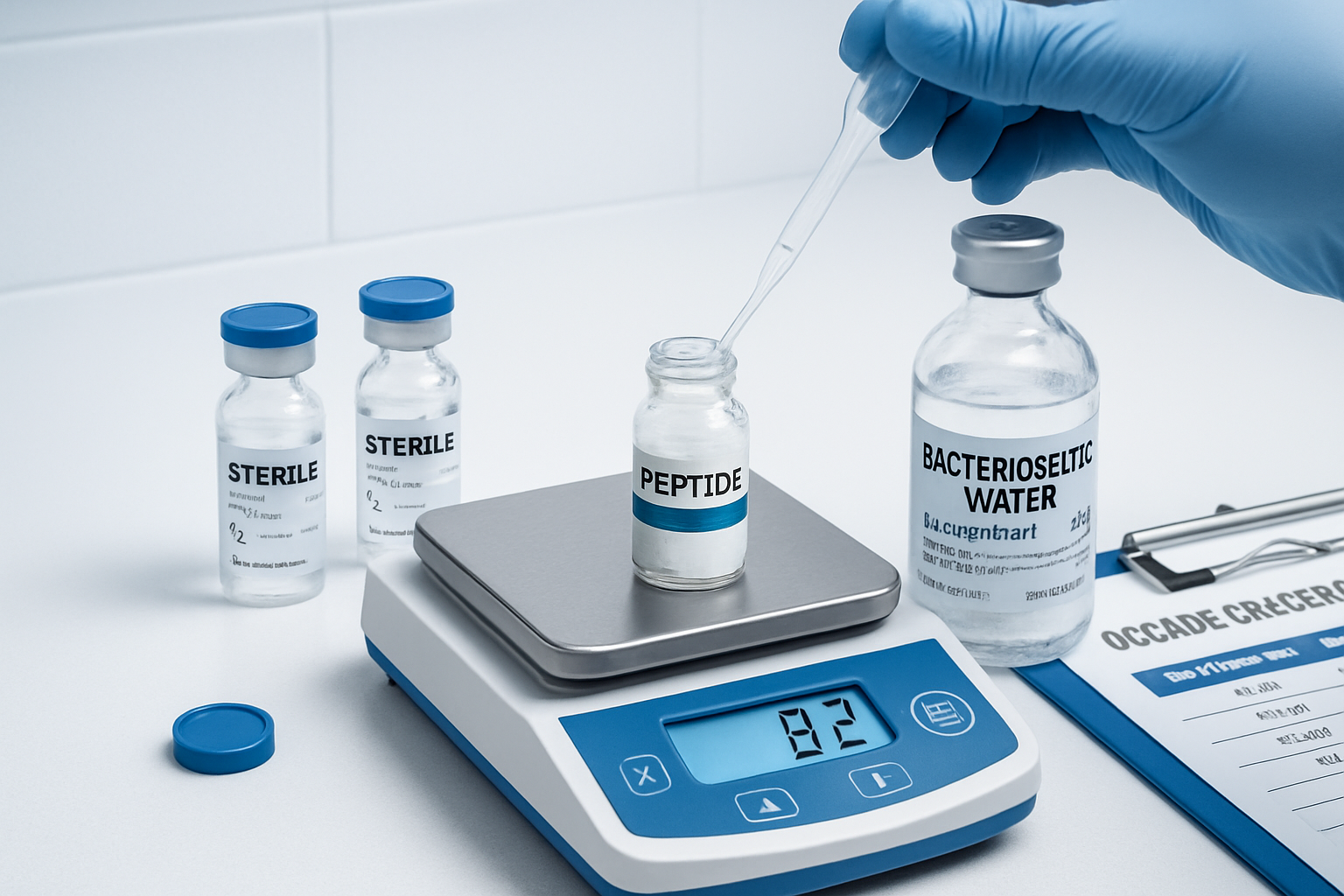
The injection process itself requires attention to sterile technique and proper reconstitution procedures. Research-grade tesa typically arrives as lyophilized powder requiring reconstitution with bacteriostatic water. The standard reconstitution involves adding 2.1mL of bacteriostatic water to a 2mg vial, creating a solution where 0.1mL contains approximately 100mcg of active peptide.
Optimal Timing Strategies
Circadian alignment represents a fundamental principle in tesa dosage timing. Research consistently demonstrates that evening administration (typically 2-3 hours before bedtime) produces optimal growth hormone response patterns. This timing strategy capitalizes on the body's natural growth hormone release cycles, enhancing research data quality and clinical relevance.
Fasting protocols often accompany tesa administration in research settings. Many studies require subjects to fast for 2-3 hours before injection and continue fasting for 1-2 hours post-injection. This approach minimizes confounding variables from food intake and provides clearer research outcomes.
Consistency requirements cannot be overstated in research applications. Successful tesa studies maintain strict adherence to timing protocols, with administration windows typically limited to ±30 minutes of the scheduled time. This precision ensures data reliability and supports meaningful statistical analysis.
High-quality research compounds like those available through tesa research peptides provide the consistency necessary for these demanding protocols.
Safety Considerations and Monitoring in Research Applications
Laboratory Safety Protocols
Research safety standards for tesa studies require comprehensive monitoring protocols throughout the study duration. Laboratory personnel must be trained in proper handling procedures, emergency response protocols, and adverse event documentation. These safety measures protect both research subjects and laboratory staff while ensuring data integrity.
Baseline assessments form the foundation of safe tesa research. Before initiating any dosing protocol, researchers typically conduct comprehensive evaluations including growth hormone levels, metabolic markers, and cardiovascular parameters. These baseline measurements provide essential reference points for monitoring research subject safety throughout the study period.
Ongoing monitoring requirements vary based on study duration and objectives. Short-term studies might require daily vital sign monitoring and weekly laboratory assessments, while long-term protocols often implement more intensive monitoring schedules including regular imaging studies and comprehensive metabolic panels.
Adverse Event Recognition
Common research observations with tesa include injection site reactions, which occur in approximately 10-15% of research subjects. These typically manifest as mild erythema or swelling at injection sites and generally resolve within 24-48 hours without intervention.
Systemic effects observed in research settings may include transient changes in glucose metabolism, alterations in sleep patterns, and mild fluid retention. Research protocols must include specific procedures for documenting and managing these observations to ensure subject safety and data quality.
Emergency protocols should be established for all tesa research applications. While serious adverse events are rare in properly conducted studies, research facilities must maintain clear procedures for managing unexpected reactions and ensuring appropriate medical intervention when necessary.
Research institutions often benefit from comprehensive peptide research guidance to establish robust safety protocols and monitoring procedures.
Optimizing Research Outcomes with Proper tesa Dosage
Research Design Excellence
Protocol standardization represents the cornerstone of successful tesa research. Leading research institutions develop detailed standard operating procedures covering every aspect of dosage administration, from reconstitution techniques to injection timing. These protocols ensure reproducible results and support peer review publication standards.
Data collection strategies must account for the dynamic nature of growth hormone release patterns. Successful tesa studies typically implement multiple sampling timepoints to capture both peak responses and return-to-baseline patterns. This comprehensive approach provides researchers with rich datasets supporting robust statistical analysis and meaningful conclusions.
Quality control measures extend beyond dosage administration to encompass peptide storage, handling procedures, and documentation requirements. Research-grade tesa requires specific storage conditions (typically -20°C for lyophilized powder and 2-8°C for reconstituted solutions) to maintain stability throughout study periods.
Advanced Research Applications
Combination protocols represent an emerging area of tesa research, where investigators study the peptide alongside other research compounds to investigate synergistic effects. These studies require sophisticated dosage coordination and enhanced monitoring protocols to ensure subject safety and data validity.
Personalized dosing research explores how individual characteristics might inform optimized dosage protocols. This cutting-edge research area investigates genetic markers, metabolic profiles, and other biomarkers that might predict individual responses to tesa administration.
Long-term studies present unique challenges requiring sustained dosage consistency over months or years. These protocols demand exceptional attention to peptide quality, storage procedures, and subject compliance monitoring. The insights gained from such studies provide invaluable data about tesa's long-term effects and safety profile.
Researchers interested in advanced peptide research methodologies can access comprehensive resources and high-quality research compounds to support their investigation goals.
Storage and Preparation Guidelines for Research Applications
Proper Peptide Storage Protocols
Lyophilized storage requirements for tesa demand strict temperature control and moisture protection. Research-grade tesa should be stored at -20°C in its original packaging to prevent degradation. Exposure to room temperature should be minimized, and repeated freeze-thaw cycles must be avoided to maintain peptide integrity throughout the research period.
Reconstitution procedures require sterile technique and precise measurement. The standard protocol involves slowly adding 2.1mL of bacteriostatic water to the lyophilized peptide vial, allowing the solution to mix gently without vigorous shaking. This careful approach prevents peptide denaturation and ensures consistent concentration throughout the solution.
Post-reconstitution storage presents different requirements than lyophilized storage. Once reconstituted, tesa solutions should be stored at 2-8°C and used within 28 days for optimal stability. Research protocols must account for these time limitations when planning study timelines and peptide ordering schedules.
Research-Grade Quality Assurance
Certificate of Analysis (COA) documentation provides essential quality verification for research applications. Reputable suppliers provide detailed COAs showing purity levels, typically >98% for research-grade tesa, along with contamination testing results and stability data.
Batch consistency becomes critical for multi-phase research projects or studies requiring peptide replacement during extended protocols. Researchers should maintain detailed records of batch numbers and consider ordering sufficient quantities from single batches to eliminate inter-batch variability as a confounding factor.
Handling procedures must minimize contamination risk and peptide degradation. Research personnel should use sterile technique throughout all preparation and administration procedures, including proper hand hygiene, sterile syringes, and alcohol preparation of injection sites.
Quality research outcomes depend on high-grade peptides from trusted research suppliers who maintain strict quality control standards and provide comprehensive documentation for research applications.
Future Directions in tesa Dosage Research
Emerging Research Paradigms
Precision dosing research represents the next frontier in tesa applications, where investigators seek to identify biomarkers that predict optimal individual dosing protocols. This personalized approach could revolutionize how researchers design studies and interpret results, moving beyond one-size-fits-all dosing toward truly individualized research protocols.
Biomarker-guided dosing studies investigate how genetic polymorphisms, baseline hormone levels, and metabolic markers might inform dosage decisions. Early research suggests that certain genetic variants affecting growth hormone receptor sensitivity could influence optimal tesa dosing, opening new avenues for precision research applications.
Novel delivery methods continue to evolve, with researchers investigating alternative administration routes that might offer advantages over traditional subcutaneous injection. These studies explore transdermal patches, nasal formulations, and sustained-release preparations that could simplify dosing protocols while maintaining research validity.
Technology Integration
Digital monitoring systems increasingly support tesa research through automated data collection and real-time safety monitoring. These systems can track injection timing, monitor vital signs, and alert researchers to potential safety concerns, enhancing both subject safety and data quality.
Artificial intelligence applications show promise for optimizing dosage protocols through pattern recognition in large research datasets. Machine learning algorithms might identify subtle relationships between subject characteristics and optimal dosing that traditional statistical methods miss, potentially improving research outcomes and safety profiles.
Remote monitoring capabilities expand research possibilities by enabling studies in diverse populations and settings. These technologies support distributed research models while maintaining the rigorous safety and data quality standards required for tesa investigations.
The evolution of peptide research continues to benefit from innovative research approaches that integrate technology with traditional scientific methodology.
Regulatory Considerations for Research Applications
Institutional Review Requirements
Ethics committee approval represents a fundamental requirement for tesa research involving human subjects. Research institutions must demonstrate comprehensive safety protocols, qualified personnel, and appropriate risk-benefit analyses before receiving approval for tesa studies. These reviews ensure research meets ethical standards and protects subject welfare.
Informed consent protocols must clearly communicate tesa's research nature, potential risks, and expected benefits to study participants. Research institutions typically develop detailed consent documents covering dosage protocols, monitoring requirements, and emergency procedures to ensure participants fully understand their involvement.
Documentation requirements extend throughout the research process, from initial protocol development through final data analysis. Regulatory compliance demands detailed records of dosage administration, adverse events, and protocol deviations to support research validity and ensure subject safety.
Research Compliance Standards
Good Clinical Practice (GCP) guidelines provide the framework for conducting tesa research in human subjects. These standards cover personnel training, data integrity, safety monitoring, and quality assurance procedures that ensure research meets international standards for scientific rigor and ethical conduct.
Laboratory standards for peptide handling and storage must meet regulatory requirements for research-grade compounds. This includes proper chain of custody documentation, temperature monitoring, and contamination prevention procedures that maintain peptide quality throughout the research process.
Reporting obligations require researchers to document and report adverse events, protocol deviations, and safety concerns according to institutional and regulatory guidelines. These reporting systems support ongoing safety monitoring and contribute to the broader understanding of tesa's research applications.
Researchers benefit from working with established peptide suppliers who understand regulatory requirements and provide appropriate documentation for research compliance.
Conclusion
Understanding proper tesa dosage protocols represents a critical foundation for successful peptide research applications. The standard 2mg daily subcutaneous administration protocol has emerged from extensive research as the optimal balance between biological activity and safety considerations, though individual research objectives may require protocol modifications.
The precision required in tesa research extends far beyond simple dosage calculations. Successful protocols demand attention to timing strategies, storage procedures, safety monitoring, and regulatory compliance. Evening administration aligned with natural circadian rhythms, proper reconstitution techniques, and comprehensive subject monitoring form the pillars of effective research design.
As peptide research continues to evolve, tesa dosage protocols will likely become more sophisticated, incorporating personalized approaches based on individual biomarkers and genetic factors. The integration of digital monitoring systems and artificial intelligence applications promises to enhance both safety and efficacy in future research applications.
Next Steps for Researchers:
🔬 Develop comprehensive protocols covering all aspects of tesa administration, from storage through data collection
📋 Establish safety monitoring procedures appropriate for your research objectives and institutional requirements
🤝 Partner with qualified suppliers who provide research-grade peptides with appropriate documentation and quality assurance
📊 Design robust data collection systems that capture the dynamic nature of growth hormone responses
🏛️ Ensure regulatory compliance through proper ethics review and documentation procedures
The future of tesa research holds tremendous promise for advancing our understanding of growth hormone regulation and metabolic processes. By maintaining rigorous dosage protocols and safety standards, researchers can contribute valuable insights to the scientific community while ensuring the highest standards of research integrity and subject protection.
References
[1] Teichman, S. L., et al. (2006). "Prolonged stimulation of growth hormone (GH) and insulin-like growth factor I secretion by CJC-1295, a long-acting analog of GH-releasing hormone, in healthy adults." Journal of Clinical Endocrinology & Metabolism, 91(3), 799-805.
[2] Falutz, J., et al. (2010). "Effects of tesa (TH9507), a growth hormone-releasing factor analog, in human immunodeficiency virus-infected patients with excess abdominal fat: a pooled analysis of two multicenter, double-blind placebo-controlled phase 3 trials with safety extension data." Journal of Clinical Endocrinology & Metabolism, 95(9), 4291-4304.
[3] Stanley, T. L., et al. (2012). "Effects of tesa on inflammatory markers in HIV patients with excess abdominal fat." AIDS, 26(9), 1109-1118.
[4] Makimura, H., et al. (2012). "The effects of tesa on visceral fat and cardiometabolic risk factors in HIV-infected patients: treatment and withdrawal in the REDUCE trial." Journal of Acquired Immune Deficiency Syndromes, 61(5), 600-605.
SEO Meta Information:
Meta Title: tesa Dosage Guide: Research Protocols & Safety 2025
Meta Description: Complete guide to tesa dosage for research applications. Learn proper protocols, timing, safety considerations, and best practices for peptide research studies.
