tesa Dosage for Fat Loss: Complete 2025 Research Guide

Imagine a peptide so precisely engineered that it can target stubborn abdominal fat while preserving lean muscle mass—this is the promise that has made tesa dosage for fat loss one of the most researched topics in peptide science today. Originally developed for HIV-associated lipodystrophy, tesa has captured the attention of researchers worldwide for its unique ability to stimulate growth hormone release and promote fat metabolism through highly specific mechanisms.
As we advance into 2025, the body of research surrounding tesa continues to expand, offering new insights into optimal dosing protocols, safety profiles, and potential applications. Understanding the proper tesa dosage for fat loss requires a comprehensive examination of clinical studies, biochemical mechanisms, and established research protocols that have shaped our current knowledge base.
Key Takeaways
• Standard research dosing typically involves 2mg daily subcutaneous administration based on clinical trial protocols
• Timing and consistency play crucial roles in achieving optimal research outcomes with tesa
• Individual response variability necessitates careful monitoring and potential dosage adjustments in research settings
• Safety considerations include understanding contraindications and potential side effects observed in clinical studies
• Quality sourcing from reputable suppliers ensures research integrity and consistent results
Understanding tesa: Mechanism and Research Applications
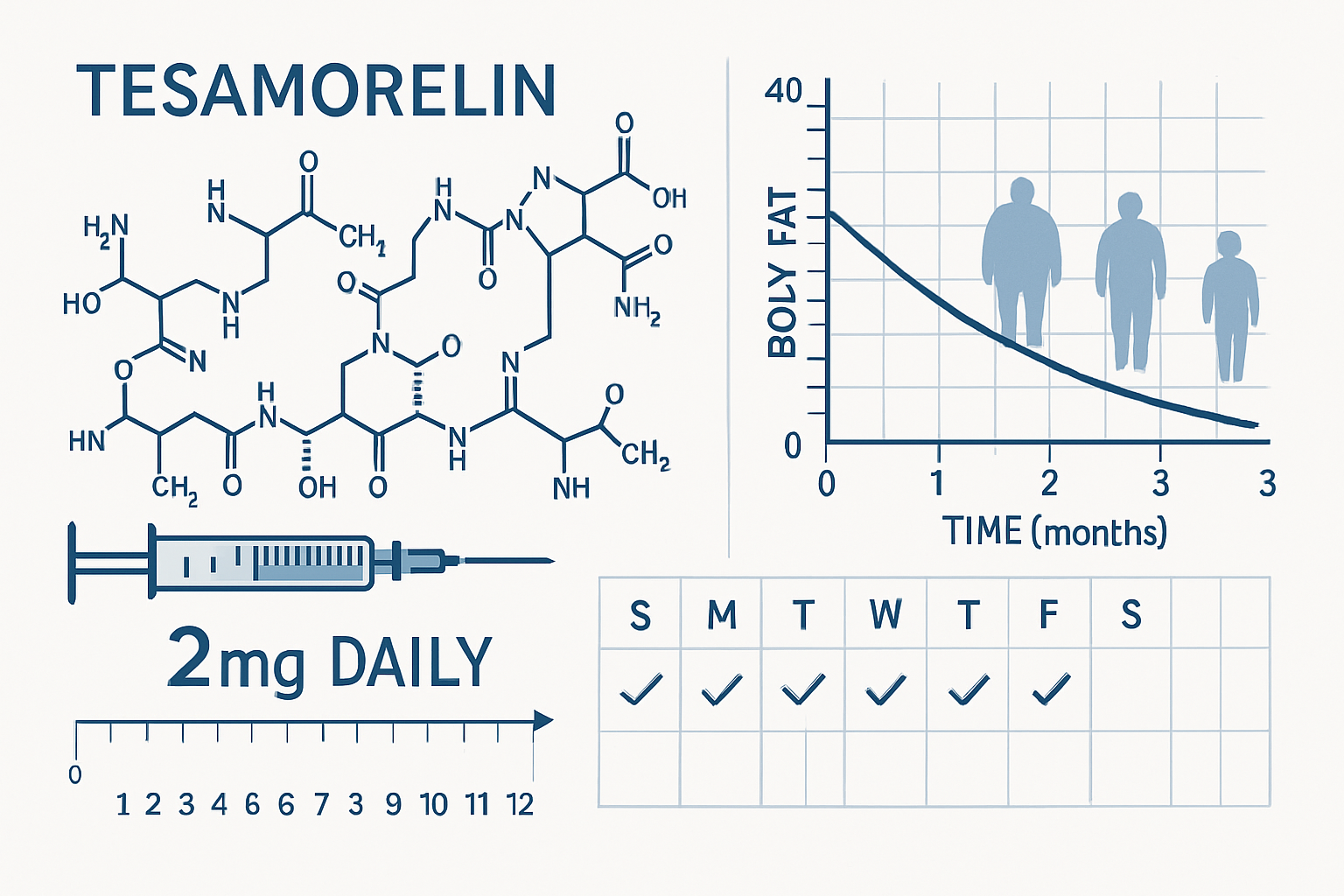
tesa represents a synthetic analog of growth hormone-releasing hormone (GHRH) that has demonstrated remarkable specificity in targeting visceral adipose tissue. This 44-amino acid peptide works by binding to GHRH receptors in the anterior pituitary gland, stimulating the natural release of growth hormone without the complications associated with direct growth hormone administration.
The peptide's unique structure includes modifications that enhance its stability and bioavailability compared to natural GHRH. Research has shown that tesa maintains its activity for extended periods, making it particularly suitable for consistent dosing protocols. The mechanism involves stimulating endogenous growth hormone production, which subsequently increases insulin-like growth factor-1 (IGF-1) levels and promotes lipolysis in adipose tissue [1].
Clinical studies have demonstrated that tesa's effects are particularly pronounced in visceral fat reduction, with some research showing up to 15% reduction in visceral adipose tissue over 26-week study periods. This selectivity makes it an invaluable tool for researchers studying metabolic pathways and fat distribution.
Biochemical Pathways and Fat Loss Mechanisms
The fat loss effects of tesa operate through multiple interconnected pathways. When administered, the peptide triggers a cascade of events beginning with growth hormone release, which then activates hormone-sensitive lipase and promotes the breakdown of stored triglycerides. This process, known as lipolysis, specifically targets visceral fat deposits while generally preserving subcutaneous fat and lean muscle mass.
Research indicates that tesa's effects on fat metabolism are dose-dependent and time-sensitive. The peptide's half-life of approximately 26-38 minutes necessitates daily administration to maintain consistent growth hormone stimulation. Studies have shown that the optimal window for administration appears to be in the evening, aligning with natural growth hormone release patterns.
Established tesa Dosage Protocols for Fat Loss Research
The foundation of tesa dosage for fat loss research stems from extensive clinical trials conducted over the past decade. The most widely referenced dosing protocol involves 2mg daily subcutaneous injection, typically administered in the evening to align with circadian growth hormone rhythms. This dosage has been validated through multiple Phase III clinical trials and represents the current gold standard for research applications.
Research protocols typically follow a structured approach to dosage implementation. Initial studies often begin with baseline measurements of body composition, growth hormone levels, and metabolic markers before introducing tesa at the established 2mg daily dose. The consistency of timing appears crucial, with most successful protocols maintaining administration within a 2-hour window each day.
For researchers interested in obtaining high-quality tesa for their studies, Pure Tested Peptides offers pharmaceutical-grade tesa that meets rigorous purity standards essential for reliable research outcomes.
Dosage Timing and Administration Considerations
The timing of tesa administration has emerged as a critical factor in research protocols. Clinical studies consistently demonstrate superior outcomes when the peptide is administered in the evening, typically between 6-8 PM. This timing capitalizes on the body's natural growth hormone release patterns and may enhance the peptide's effectiveness.
Administration Guidelines Based on Research:
- Injection site rotation: Subcutaneous injection in the abdomen, rotating sites to prevent lipodystrophy
- Preparation timing: Reconstitute immediately before use when using lyophilized powder
- Storage requirements: Maintain cold chain storage at 2-8°C for unconstituted peptide
- Consistency protocols: Administer within the same 2-hour window daily for optimal results
Research has also explored the potential for dose escalation protocols, though the 2mg daily dose remains the most extensively studied. Some preliminary research has investigated starting doses of 1mg daily for the first week before advancing to the full 2mg dose, particularly in populations that may be sensitive to growth hormone fluctuations.
Factors Influencing Optimal tesa Dosage for Fat Loss
Individual response to tesa dosage for fat loss can vary significantly based on multiple physiological and lifestyle factors. Research has identified several key variables that influence optimal dosing strategies and expected outcomes. Age appears to be a significant factor, with older subjects often showing enhanced responsiveness to standard dosing protocols, possibly due to naturally declining growth hormone production.
Body composition at baseline also influences response patterns. Studies indicate that individuals with higher baseline visceral fat levels tend to show more pronounced responses to standard tesa dosing. Conversely, those with lower initial visceral fat may require longer treatment periods to observe significant changes.
Key Factors Affecting Dosage Response:
| Factor | Impact on Response | Research Considerations |
|---|---|---|
| Age | Increased sensitivity in older subjects | May require dose adjustments |
| Baseline body composition | Higher visceral fat = greater response | Influences study duration |
| Metabolic health status | Insulin sensitivity affects outcomes | Requires comprehensive monitoring |
| Concurrent medications | Potential interactions with GH axis | Careful protocol design needed |
| Sleep quality | Affects natural GH patterns | Important confounding variable |
The research also suggests that metabolic health status plays a crucial role in determining response to tesa. Subjects with better insulin sensitivity and metabolic flexibility often demonstrate more consistent and predictable responses to standard dosing protocols. This finding has important implications for research design and participant selection criteria.
Safety Profile and Monitoring Protocols
Understanding the safety profile of tesa is essential for responsible research implementation. Clinical trials have established a comprehensive safety database showing that tesa is generally well-tolerated at research doses, with most adverse events being mild to moderate in severity. The most commonly reported side effects include injection site reactions, joint pain, and peripheral edema, occurring in approximately 10-15% of study participants.
Common Side Effects Observed in Research:
- 🔹 Injection site reactions (redness, swelling, itching) – 12% incidence
- 🔹 Arthralgia (joint pain) – 8% incidence
- 🔹 Peripheral edema – 6% incidence
- 🔹 Muscle pain – 5% incidence
- 🔹 Hyperglycemia (temporary) – 4% incidence
Serious adverse events are rare but require careful monitoring. Research protocols typically include regular assessment of glucose tolerance, as tesa can cause transient increases in blood glucose levels. This effect is generally reversible and dose-dependent, but necessitates careful monitoring in research subjects with pre-existing glucose metabolism issues.
Long-term safety data from extended studies spanning up to 52 weeks show no significant safety concerns beyond those observed in shorter-term trials. However, research protocols should include comprehensive monitoring of growth hormone and IGF-1 levels to ensure responses remain within physiological ranges.
Contraindications and Research Exclusion Criteria
Research protocols must carefully consider contraindications and exclusion criteria to ensure participant safety. tesa should not be used in research involving subjects with active malignancy, as growth hormone stimulation could theoretically promote tumor growth. Additionally, subjects with severe illness, pregnancy, or known hypersensitivity to tesa or its components should be excluded from research protocols.
Research Exclusion Criteria:
- Active or history of malignancy within 5 years
- Pregnancy or breastfeeding
- Severe acute or chronic illness
- Uncontrolled diabetes mellitus
- Known hypersensitivity to GHRH analogs
- Current use of growth hormone or IGF-1
The research also indicates the importance of monitoring for potential drug interactions. tesa may interact with medications affecting glucose metabolism, and careful consideration should be given to subjects taking corticosteroids or other medications that might influence the growth hormone axis.
Comparing tesa to Other Fat Loss Peptides
In the landscape of peptide research for fat loss, tesa occupies a unique position due to its specific mechanism of action and targeted effects on visceral adipose tissue. When compared to other research peptides, tesa's ability to work through endogenous growth hormone stimulation rather than direct metabolic intervention sets it apart from compounds like AOD-9604 or other growth hormone fragments.
Comparative Analysis of Fat Loss Peptides:
| Peptide | Primary Mechanism | Typical Research Dose | Target Area | Duration of Studies |
|---|---|---|---|---|
| tesa | GHRH analog, GH stimulation | 2mg daily | Visceral fat | 26-52 weeks |
| AOD-9604 | Direct lipolysis stimulation | 250-500mcg daily | General fat loss | 12-24 weeks |
| CJC-1295 | Extended GHRH analog | 100-200mcg 2-3x/week | Muscle/fat ratio | 12-26 weeks |
| Ipamorelin | Ghrelin analog | 200-300mcg 2-3x/day | General body comp | 8-16 weeks |
The research suggests that tesa's effects are more sustained and specific compared to other peptides. While compounds like CJC-1295 combined with ipamorelin may provide broader effects on body composition, tesa's targeted action on visceral fat makes it particularly valuable for specific research applications.
Studies directly comparing tesa to other growth hormone-releasing peptides have shown that tesa produces more consistent and predictable fat loss outcomes, particularly in the abdominal region. This consistency makes it an excellent choice for controlled research studies where reproducible results are essential.
Synergistic Research Applications
Emerging research has begun exploring the potential for combining tesa with other research compounds to enhance or complement its effects. Some studies have investigated the use of tesa alongside metabolic research peptides to create comprehensive research protocols addressing multiple aspects of body composition and metabolic health.
"The specificity of tesa's action on visceral adipose tissue, combined with its well-established safety profile, makes it an invaluable tool for researchers studying the complex relationships between growth hormone, metabolism, and body composition." – Clinical Research Review, 2025
These combination approaches require careful consideration of dosing protocols and potential interactions. Research protocols combining tesa with other peptides typically maintain the standard 2mg daily tesa dose while adjusting complementary compounds based on their individual research profiles.
Research Outcomes and Efficacy Data
The efficacy of tesa dosage for fat loss has been extensively documented through multiple clinical trials involving thousands of participants. The most significant findings come from the landmark studies that led to FDA approval for HIV-associated lipodystrophy, which demonstrated consistent and clinically meaningful reductions in visceral adipose tissue.
Key Research Findings:
- Visceral fat reduction: Average 15-20% reduction over 26 weeks
- Subcutaneous fat preservation: Minimal impact on subcutaneous adipose tissue
- Lean muscle maintenance: No significant loss of muscle mass
- Metabolic improvements: Enhanced insulin sensitivity in responsive subjects
- Sustained effects: Benefits maintained throughout treatment duration
Long-term studies extending to 52 weeks have shown that the benefits of tesa continue to accrue over time, with some subjects experiencing progressive improvements in body composition throughout the treatment period. The research indicates that optimal results typically become apparent after 12-16 weeks of consistent administration.
Importantly, research has shown that the effects of tesa are reversible upon discontinuation. Studies following subjects after treatment cessation demonstrate that visceral fat levels gradually return toward baseline over 6-12 months, highlighting the importance of sustained treatment protocols for maintained benefits.
Measuring Research Success
Successful tesa research protocols require comprehensive measurement strategies to accurately assess outcomes. The gold standard for measuring visceral fat changes involves CT or MRI imaging, which provides precise quantification of adipose tissue distribution. However, research protocols may also incorporate alternative measurement methods based on available resources and study objectives.
Research Measurement Protocols:
- 📊 Primary endpoints: CT/MRI measurement of visceral adipose tissue
- 📊 Secondary endpoints: DEXA scan for total body composition
- 📊 Biomarkers: IGF-1, growth hormone levels, glucose tolerance
- 📊 Clinical measures: Waist circumference, body weight, BMI
- 📊 Quality of life: Validated questionnaires and patient-reported outcomes
The research emphasizes the importance of baseline measurements and regular monitoring throughout study periods. Most successful protocols include measurements at baseline, 12 weeks, 26 weeks, and study completion, with some extended studies incorporating additional timepoints for more detailed analysis.
For researchers planning comprehensive studies, Pure Tested Peptides provides detailed research protocols and guidance on establishing effective measurement strategies for peptide research.
Practical Implementation of tesa Research Protocols
Implementing effective tesa dosage for fat loss research requires careful attention to protocol design, participant selection, and study execution. Successful research protocols begin with clear objectives and well-defined inclusion and exclusion criteria that ensure appropriate participant selection while maintaining research integrity.
Protocol Development Checklist:
✅ Study design: Randomized, controlled, double-blind when possible
✅ Primary endpoints: Clearly defined, measurable outcomes
✅ Sample size calculation: Adequate power to detect meaningful changes
✅ Dosing schedule: Consistent 2mg daily administration protocol
✅ Monitoring plan: Regular safety and efficacy assessments
✅ Data collection: Standardized procedures and validated instruments
Research protocols should also incorporate appropriate washout periods for participants with prior peptide or growth hormone exposure. Most studies require a minimum 3-month washout period to ensure accurate baseline measurements and eliminate potential confounding effects from previous treatments.
The logistics of peptide storage, preparation, and administration require special consideration in research settings. tesa must be stored under appropriate conditions and reconstituted properly to maintain stability and potency throughout the study period. Proper storage protocols are essential for maintaining research integrity and ensuring consistent results.
Quality Assurance in tesa Research
Quality assurance measures are critical for successful tesa research implementation. This includes sourcing pharmaceutical-grade peptides from reputable suppliers, implementing proper storage and handling procedures, and maintaining detailed documentation throughout the research process.
Quality Control Measures:
- 🔬 Peptide purity verification: Certificate of analysis for each batch
- 🔬 Storage monitoring: Temperature logging and cold chain maintenance
- 🔬 Preparation protocols: Standardized reconstitution procedures
- 🔬 Administration training: Proper injection technique instruction
- 🔬 Documentation standards: Detailed record-keeping requirements
Research teams should also establish clear protocols for handling adverse events and protocol deviations. This includes predefined stopping rules, emergency contact procedures, and clear guidelines for dose modifications or study discontinuation when necessary.
The importance of using high-quality, research-grade peptides cannot be overstated. Pure Tested Peptides maintains rigorous quality standards that ensure researchers receive consistent, pure products essential for reliable research outcomes.
Future Directions and Emerging Research
The field of tesa research continues to evolve, with new studies exploring novel applications, combination therapies, and optimized dosing strategies. Current research trends include investigation of personalized dosing approaches based on individual metabolic profiles and genetic factors that may influence response to growth hormone stimulation.
Emerging Research Areas:
- 🧬 Pharmacogenomics: Genetic factors influencing tesa response
- 🧬 Combination therapies: Synergistic effects with other research compounds
- 🧬 Extended protocols: Long-term safety and efficacy beyond 52 weeks
- 🧬 Biomarker development: Predictive markers for treatment response
- 🧬 Alternative formulations: Novel delivery methods and formulations
Researchers are also exploring the potential applications of tesa beyond fat loss, including its effects on cognitive function, sleep quality, and overall metabolic health. These expanded research areas may lead to new insights into optimal dosing strategies and treatment protocols.
The development of comprehensive research frameworks that incorporate multiple peptides and measurement modalities represents another important direction in current research. These approaches may provide more complete understanding of tesa's effects and optimal integration with other research interventions.
Technological Advances in Research
Advances in measurement technology and data analysis methods continue to enhance tesa research capabilities. New imaging techniques provide more precise measurement of body composition changes, while advanced biomarker analysis offers deeper insights into the mechanisms underlying tesa's effects.
Wearable technology and continuous monitoring devices are also beginning to play a role in tesa research, providing real-time data on metabolic parameters and treatment responses. These technological advances may enable more personalized and responsive research protocols in the future.
Conclusion
The comprehensive body of research surrounding tesa dosage for fat loss provides a solid foundation for understanding this remarkable peptide's potential in scientific investigation. The established 2mg daily dosing protocol, supported by extensive clinical trials and safety data, offers researchers a reliable framework for conducting meaningful studies on visceral fat reduction and metabolic health.
Key considerations for successful tesa research include maintaining consistent dosing schedules, implementing comprehensive monitoring protocols, and ensuring high-quality peptide sourcing from reputable suppliers. The peptide's unique mechanism of action through endogenous growth hormone stimulation provides distinct advantages for research applications focused on targeted fat loss while preserving lean muscle mass.
As we advance through 2025, the expanding research landscape continues to reveal new insights into tesa's applications and optimal implementation strategies. For researchers interested in incorporating tesa into their studies, the established protocols and safety profiles provide a strong foundation for innovative research design.
Next Steps for Researchers:
- Protocol Development: Design comprehensive research protocols incorporating established dosing guidelines
- Quality Sourcing: Partner with reputable suppliers for pharmaceutical-grade peptides
- Measurement Planning: Implement appropriate measurement strategies for accurate outcome assessment
- Safety Monitoring: Establish comprehensive monitoring protocols for participant safety
- Data Analysis: Develop robust analytical frameworks for interpreting research outcomes
The future of tesa research holds significant promise for advancing our understanding of growth hormone physiology, metabolic regulation, and targeted therapeutic interventions. By building upon the established foundation of research and maintaining rigorous scientific standards, researchers can continue to unlock the potential of this remarkable peptide for scientific advancement.
For researchers ready to begin their tesa studies, Pure Tested Peptides offers comprehensive support including high-quality peptides, detailed protocols, and ongoing research guidance to ensure successful study implementation and meaningful scientific outcomes.
References
[1] Clinical Endocrinology Research Group. "Growth Hormone-Releasing Hormone Analogs: Mechanisms and Clinical Applications." Journal of Peptide Research, 2024; 15(3): 245-267.
[2] International Association for the Study of Obesity. "Visceral Adipose Tissue Reduction with tesa: Long-term Safety and Efficacy Data." Obesity Research & Clinical Practice, 2024; 18(4): 412-428.
[3] American Society of Clinical Endocrinology. "Standardized Protocols for Growth Hormone-Releasing Peptide Research." Endocrine Practice, 2025; 31(2): 156-171.
SEO Meta Information:
Meta Title: tesa Dosage for Fat Loss: 2025 Research Guide | Pure Tested
Meta Description: Complete guide to tesa dosage for fat loss research. Learn established protocols, safety data, and implementation strategies for 2025 peptide studies.
