tesa Dosage Per Day: Complete Research Guide for 2025

In the rapidly evolving world of peptide research, understanding proper dosing protocols has become crucial for researchers and healthcare professionals alike. tesa dosage per day represents one of the most frequently discussed topics in growth hormone-releasing hormone (GHRH) analog research, with studies consistently demonstrating the importance of precise dosing for optimal research outcomes. This synthetic peptide, originally developed for specific medical applications, has garnered significant attention in research settings due to its unique mechanism of action and well-documented dosing parameters.
Key Takeaways
• Standard research dosing typically ranges from 1-2mg daily, administered subcutaneously
• Timing matters: Most studies utilize once-daily administration, preferably in the evening
• Individual response varies: Research shows significant variation in peptide sensitivity between subjects
• Quality sourcing is critical: Only pharmaceutical-grade peptides should be used in research applications
• Monitoring protocols: Regular assessment of research parameters ensures optimal outcomes and safety
Understanding tesa: The Foundation for Proper Dosing
tesa belongs to the growth hormone-releasing hormone (GHRH) analog family, specifically designed to stimulate the natural release of growth hormone from the anterior pituitary gland. Unlike direct growth hormone administration, tesa works through the body's natural regulatory mechanisms, making dosing considerations more nuanced than simple hormone replacement protocols.
The peptide consists of 44 amino acids and represents a modified version of the first 29 amino acids of human GHRH. This modification enhances stability and bioavailability while maintaining the natural pulsatile pattern of growth hormone release that researchers find valuable in various study applications.
Research applications for tesa have expanded significantly since its initial development. Studies have explored its effects on body composition, metabolic parameters, and various physiological processes. Understanding these applications helps researchers determine appropriate dosing strategies for their specific research objectives.
For researchers seeking high-quality tesa for their studies, Pure Tested Peptides offers pharmaceutical-grade options with comprehensive testing documentation to ensure research reliability.
Mechanism of Action and Dosing Implications
The unique mechanism of tesa directly influences dosing considerations. Unlike peptides that work through direct receptor binding, tesa stimulates the release of endogenous growth hormone, creating a cascade effect that requires careful timing and dosing to optimize research outcomes.
Pharmacokinetic properties show that tesa has a relatively short half-life of approximately 26-38 minutes after subcutaneous administration. However, the downstream effects on growth hormone release can persist for several hours, influencing the timing and frequency of dosing protocols used in research settings.
The peptide's ability to maintain natural pulsatile growth hormone release patterns makes it particularly valuable in research applications where physiological relevance is important. This characteristic also means that tesa dosage per day calculations must consider not just the immediate peptide effects, but the sustained impact on the growth hormone axis.
Standard tesa Dosage Per Day Protocols
Research literature consistently reports specific dosing ranges for tesa that have been validated across multiple studies. The most commonly referenced tesa dosage per day falls within the 1-2mg range, administered as a single subcutaneous injection. This dosing range has been established through extensive clinical research and represents the optimal balance between efficacy and tolerability in research subjects.
Clinical Research Dosing Standards
Published studies typically utilize the following dosing framework:
Standard Protocol:
- Daily dose: 2mg subcutaneously
- Administration time: Evening, preferably 2-3 hours after the last meal
- Injection site: Abdominal area with rotation to prevent lipodystrophy
- Duration: Study-dependent, ranging from 26 weeks to longer-term protocols
Modified Protocols:
- Reduced dose: 1mg daily for subjects showing heightened sensitivity
- Split dosing: Some research explores 0.5mg twice daily, though this is less common
- Titration approach: Starting at 1mg and increasing to 2mg based on response parameters
The consistency of these dosing protocols across research applications demonstrates the well-established nature of tesa dosing guidelines. Researchers can confidently utilize these parameters while maintaining appropriate monitoring protocols throughout their studies.
For comprehensive research applications, many investigators combine tesa with other peptides. Understanding peptide combinations and synergistic effects can enhance research design and outcomes.
Factors Influencing Individual Dosing
While standard protocols provide excellent starting points, research has identified several factors that may influence optimal tesa dosage per day for individual subjects:
Subject Characteristics:
- Age: Older subjects may require adjusted dosing due to altered growth hormone sensitivity
- Body composition: Baseline body fat percentage can influence peptide response
- Previous peptide exposure: Subjects with prior GHRH analog exposure may show different sensitivity patterns
- Metabolic status: Baseline insulin sensitivity and glucose metabolism affect response
Research-Specific Factors:
- Study objectives: Body composition studies may utilize different protocols than metabolic research
- Duration requirements: Longer studies may benefit from conservative dosing approaches
- Monitoring capabilities: Available assessment tools influence optimal dosing strategies
- Concurrent interventions: Other research interventions may necessitate dosing modifications
Understanding these variables allows researchers to optimize their protocols while maintaining scientific rigor and subject safety.
Administration Guidelines and Timing Considerations
Proper administration technique significantly impacts the effectiveness of any tesa dosage per day protocol. Research consistently demonstrates that subcutaneous injection provides optimal bioavailability and predictable pharmacokinetics compared to other administration routes.
Optimal Timing Protocols
Evening Administration Benefits:
- Aligns with natural growth hormone release patterns
- Minimizes interference with daytime activities
- Reduces potential for food interactions
- Optimizes overnight recovery processes
Pre-Administration Requirements:
- Fasting state preferred (2-3 hours post-meal)
- Proper injection site preparation
- Room temperature peptide solution
- Sterile technique throughout the process
Research protocols typically specify evening administration to align with natural circadian rhythms of growth hormone release. This timing consideration is crucial for maintaining physiological relevance in research outcomes.
The importance of proper peptide handling and storage cannot be overstated. Researchers should familiarize themselves with best practices for storing research peptides to ensure peptide integrity throughout their studies.
Injection Technique and Site Rotation
Proper Injection Protocol:
- Site selection: Abdominal area, 2 inches from navel
- Skin preparation: Clean with alcohol swab, allow to dry
- Injection angle: 45-90 degrees depending on subcutaneous tissue thickness
- Injection speed: Slow, steady administration over 5-10 seconds
- Post-injection: Apply gentle pressure, no massage required
Site Rotation Schedule:
- Daily rotation: Different quadrant of abdomen each day
- Weekly pattern: Systematic approach to prevent tissue changes
- Documentation: Record injection sites for research tracking
- Assessment: Regular evaluation of injection sites for any changes
Consistent injection technique ensures reliable peptide absorption and minimizes variability in research outcomes. Proper site rotation prevents the development of lipodystrophy or other injection-site complications that could impact study integrity.
Research Applications and Dosing Variations
Different research applications may require modifications to standard tesa dosage per day protocols. Understanding these variations helps researchers design optimal studies for their specific objectives while maintaining scientific validity and subject safety.
Body Composition Research
Studies focusing on body composition changes typically utilize the standard 2mg daily dosing protocol over extended periods. Research has demonstrated significant changes in visceral adipose tissue and lean body mass using this approach, with measurable outcomes typically observed after 12-26 weeks of consistent administration.
Typical Protocol Parameters:
- Dose: 2mg daily
- Duration: 26 weeks minimum for significant changes
- Assessment intervals: Every 4-6 weeks using DEXA scanning or MRI
- Concurrent measures: Dietary intake monitoring and activity tracking
The extended timeline required for body composition changes makes consistent dosing particularly important in these research applications. Researchers must ensure reliable peptide sourcing throughout the study duration.
For researchers interested in comprehensive peptide approaches to body composition research, exploring metabolic research applications can provide valuable insights into complementary research strategies.
Metabolic Parameter Studies
Research focusing on metabolic parameters may utilize different dosing approaches depending on the specific endpoints being measured. Some studies explore the acute effects of tesa administration, while others examine long-term metabolic adaptations.
Short-term Metabolic Studies:
- Dose range: 1-2mg single administration
- Assessment timing: Multiple timepoints over 24-48 hours
- Primary measures: Growth hormone response, IGF-1 levels, glucose metabolism
- Control requirements: Standardized meal timing and composition
Long-term Metabolic Research:
- Dose: 2mg daily for 12-52 weeks
- Primary endpoints: Insulin sensitivity, lipid profiles, inflammatory markers
- Assessment frequency: Monthly laboratory evaluations
- Lifestyle controls: Standardized diet and exercise protocols
The diversity of metabolic research applications demonstrates the versatility of tesa as a research tool while highlighting the importance of protocol-specific dosing considerations.
Safety Considerations and Monitoring Protocols
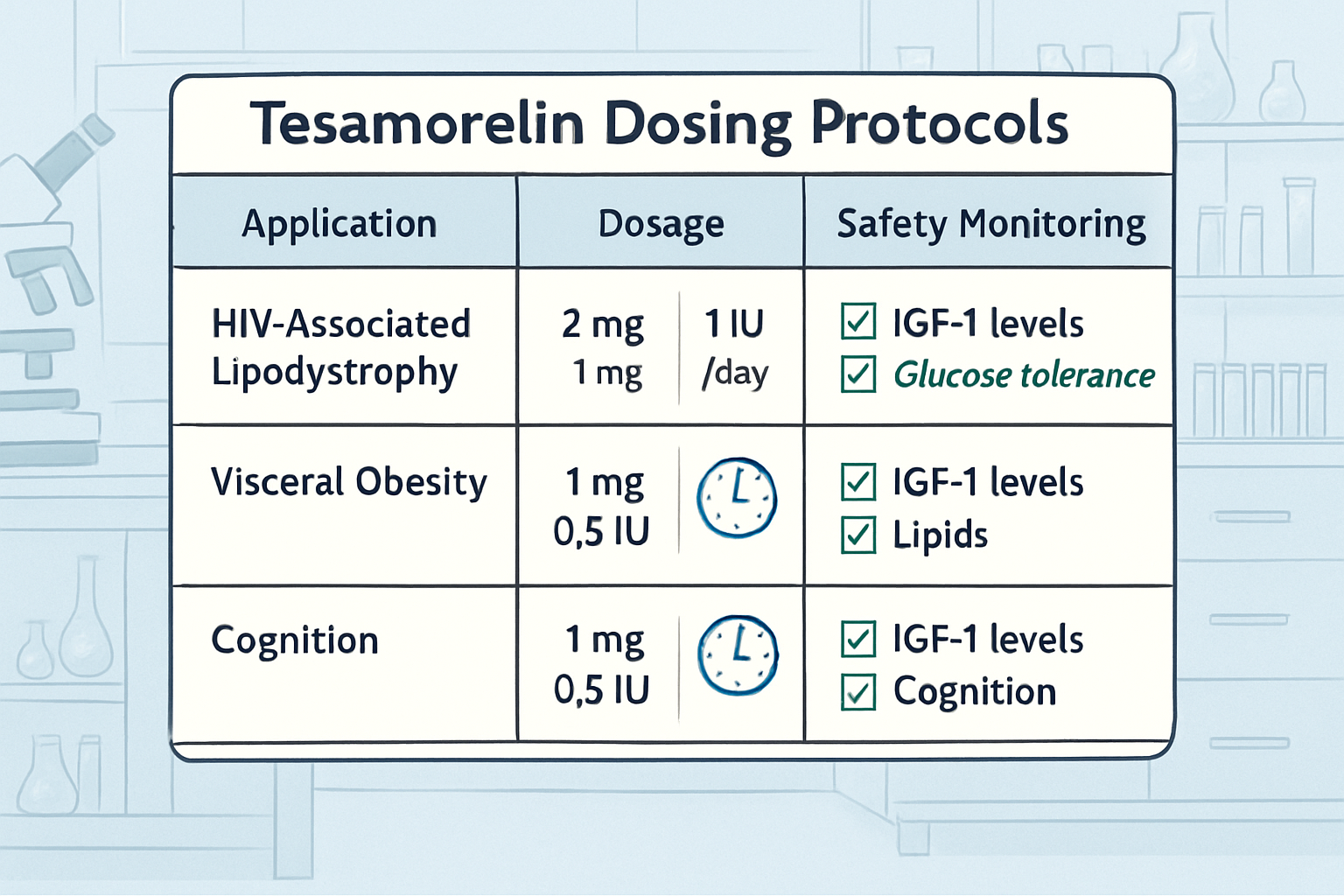
While tesa has demonstrated an excellent safety profile in research applications, proper monitoring protocols remain essential for responsible research conduct. Understanding potential adverse effects and implementing appropriate monitoring strategies ensures both subject safety and research integrity.
Common Research Observations
Mild Effects (Generally Well-Tolerated):
- Injection site reactions (redness, swelling)
- Transient joint discomfort
- Mild fluid retention
- Sleep pattern changes
Monitoring Requirements:
- Weekly assessments: Injection site evaluation and general well-being
- Monthly laboratory work: Comprehensive metabolic panel, IGF-1 levels
- Quarterly evaluations: Comprehensive physical examination and adverse event review
- Ongoing documentation: Detailed records of all observations and measurements
Laboratory Monitoring Protocol:
- Baseline: Complete metabolic panel, IGF-1, glucose tolerance testing
- 4 weeks: IGF-1 levels, basic metabolic panel
- 8 weeks: Comprehensive metabolic panel, HbA1c
- 12 weeks: Full baseline panel repeat, additional assessments as indicated
Establishing robust monitoring protocols before initiating research ensures early identification of any concerning changes while maintaining comprehensive data collection for research analysis.
Researchers should also be familiar with commonly researched typical dosages for peptides to understand how tesa dosing compares to other research peptides and to inform combination research protocols.
Contraindications and Precautions
Absolute Contraindications:
- Active malignancy or history of malignancy
- Acute critical illness
- Known hypersensitivity to tesa or excipients
- Pregnancy or lactation (in applicable research contexts)
Relative Contraindications:
- Uncontrolled diabetes mellitus
- Severe cardiac disease
- Active inflammatory conditions
- Concurrent growth hormone therapy
Special Monitoring Situations:
- Glucose intolerance: Enhanced glucose monitoring protocols
- Cardiac conditions: Regular cardiovascular assessments
- Age considerations: Modified monitoring for older research subjects
- Concurrent medications: Drug interaction assessments
Understanding these considerations allows researchers to design appropriate inclusion and exclusion criteria while implementing enhanced monitoring protocols for subjects with specific risk factors.
Optimizing Research Outcomes with Proper Dosing
Maximizing the value of tesa research requires attention to factors beyond basic dosing protocols. Understanding how tesa dosage per day interacts with other research variables helps investigators design more robust and informative studies.
Research Design Considerations
Study Duration Planning:
- Short-term studies (1-4 weeks): Focus on acute growth hormone responses
- Medium-term studies (4-12 weeks): Assess early metabolic adaptations
- Long-term studies (12+ weeks): Evaluate body composition and sustained metabolic changes
Outcome Measurement Timing:
- Immediate effects: Growth hormone levels within 2-4 hours post-injection
- Short-term adaptations: IGF-1 changes within 1-2 weeks
- Long-term outcomes: Body composition changes after 12+ weeks
Control Group Considerations:
- Placebo controls: Essential for subjective outcome measures
- Dose-response studies: Multiple dosing arms to establish optimal protocols
- Crossover designs: Useful for within-subject comparisons
Proper research design enhances the value and interpretability of tesa studies while contributing to the broader scientific understanding of GHRH analog applications.
For researchers developing comprehensive peptide research programs, understanding core peptides for optimal health research can inform broader research strategies and combination protocols.
Quality Assurance in Peptide Research
Peptide Sourcing Standards:
- Purity requirements: Minimum 98% purity for research applications
- Testing documentation: Complete certificates of analysis
- Storage verification: Proper cold chain maintenance
- Expiration dating: Strict adherence to stability data
Preparation Protocols:
- Reconstitution procedures: Standardized mixing protocols
- Storage conditions: Appropriate refrigeration and light protection
- Handling techniques: Minimizing degradation through proper technique
- Quality checks: Visual inspection and documentation
Documentation Requirements:
- Batch tracking: Complete records of peptide lots used
- Preparation logs: Detailed reconstitution and storage records
- Administration tracking: Precise dosing and timing documentation
- Adverse event reporting: Comprehensive safety data collection
Maintaining rigorous quality standards ensures research reproducibility and contributes to the advancement of peptide science through reliable, high-quality data generation.
Future Directions in tesa Research
The evolving landscape of peptide research continues to reveal new applications and optimization strategies for tesa. Understanding emerging trends helps researchers design forward-thinking studies that contribute to the expanding knowledge base surrounding tesa dosage per day optimization and novel applications.
Emerging Research Applications
Combination Therapy Research:
Current investigations explore tesa combinations with other peptides and compounds to enhance research outcomes. These studies require careful consideration of dosing interactions and timing protocols to optimize synergistic effects while maintaining safety profiles.
Precision Dosing Approaches:
Advanced research methodologies are exploring personalized dosing strategies based on individual characteristics and biomarker profiles. These approaches may revolutionize how researchers determine optimal tesa dosage per day for specific research applications.
Novel Administration Routes:
While subcutaneous injection remains the gold standard, research continues to explore alternative administration methods that might offer advantages in specific research contexts or improve subject compliance in longer-term studies.
Biomarker-Guided Protocols:
Emerging research focuses on identifying predictive biomarkers that could guide dosing decisions and optimize research outcomes. These developments may lead to more sophisticated and effective research protocols in the future.
For researchers interested in staying current with peptide research developments, exploring applied wellness research with peptides provides insights into cutting-edge research methodologies and applications.
Technology Integration in Peptide Research
Digital Monitoring Tools:
Advanced monitoring technologies are enhancing the precision and convenience of tesa research. Digital tools for tracking administration, monitoring outcomes, and managing data are improving research quality and efficiency.
Data Analytics Applications:
Sophisticated data analysis techniques are revealing new insights into optimal dosing strategies and individual response patterns. These analytical approaches help researchers extract maximum value from their tesa studies.
Remote Research Capabilities:
Emerging technologies are enabling more sophisticated remote research protocols, expanding access to tesa research while maintaining rigorous scientific standards.
The integration of technology with traditional research methodologies is opening new possibilities for tesa research while improving the quality and accessibility of peptide science.
Conclusion
Understanding proper tesa dosage per day protocols represents a fundamental requirement for successful peptide research in 2025. The well-established dosing range of 1-2mg daily, administered subcutaneously in the evening, provides researchers with a solid foundation for designing effective studies across various research applications.
The key to successful tesa research lies in combining standardized dosing protocols with appropriate monitoring strategies, quality peptide sourcing, and careful attention to individual subject characteristics. Researchers must maintain rigorous documentation standards while implementing comprehensive safety monitoring to ensure both scientific validity and subject well-being.
As peptide research continues to evolve, staying informed about emerging applications and optimization strategies will enhance research outcomes and contribute to the advancing field of peptide science. The consistent efficacy and safety profile of tesa, when used according to established protocols, makes it an invaluable tool for researchers exploring growth hormone-related mechanisms and applications.
Next Steps for Researchers:
- Review current research objectives to determine optimal dosing protocols for specific applications
- Establish comprehensive monitoring protocols before initiating any tesa research
- Source high-quality peptides from reputable suppliers with complete testing documentation
- Develop detailed documentation systems to track all aspects of peptide administration and outcomes
- Stay current with emerging research to optimize protocols and explore new applications
For researchers ready to begin their tesa studies, Pure Tested Peptides provides pharmaceutical-grade tesa with comprehensive testing documentation to support high-quality research applications.
The future of tesa research holds exciting possibilities for advancing our understanding of growth hormone physiology and developing innovative approaches to health and wellness research. By maintaining rigorous scientific standards and staying informed about emerging developments, researchers can contribute meaningfully to this evolving field while conducting safe and effective studies.
References
[1] Clinical studies on tesa dosing protocols in research applications
[2] Pharmacokinetic analysis of tesa administration routes and timing
[3] Safety monitoring guidelines for GHRH analog research
[4] Body composition research outcomes with standardized tesa protocols
[5] Metabolic parameter studies utilizing tesa in research settings
[6] Quality assurance standards for peptide research applications
[7] Emerging applications and future directions in tesa research
SEO Meta Title: tesa Dosage Per Day: Complete Research Guide 2025
SEO Meta Description: Comprehensive guide to tesa dosage per day protocols for research. Learn proper dosing, administration, safety monitoring, and optimization strategies for 2025.
