cjc1295/ipamorelin;390
CJC1295/Ipamorelin;390: Complete Guide to This Peptide Combination in 2025
Imagine unlocking your body's natural potential through cutting-edge peptide research that has captivated scientists worldwide. The combination of cjc1295/ipamorelin;390 represents one of the most studied peptide protocols in growth hormone research, offering fascinating insights into how these compounds work synergistically at the cellular level.
This powerful peptide combination has emerged as a focal point in laboratory studies examining growth hormone releasing mechanisms. Research into cjc1295 and ipamorelin individually has paved the way for understanding their combined effects, making cjc1295/ipamorelin;390 a subject of intense scientific interest.
Key Takeaways
• cjc1295/ipamorelin;390 combines two distinct peptides that work through different pathways to stimulate growth hormone release
• Laboratory studies show this combination may offer synergistic effects compared to individual peptide use
• The "390" designation refers to specific dosing protocols used in research settings
• Both peptides have been extensively studied for their stability and mechanism of action
• Research suggests this combination may offer improved bioavailability and sustained effects
Understanding CJC1295/Ipamorelin;390 Fundamentals
What Makes This Combination Unique? 🔬
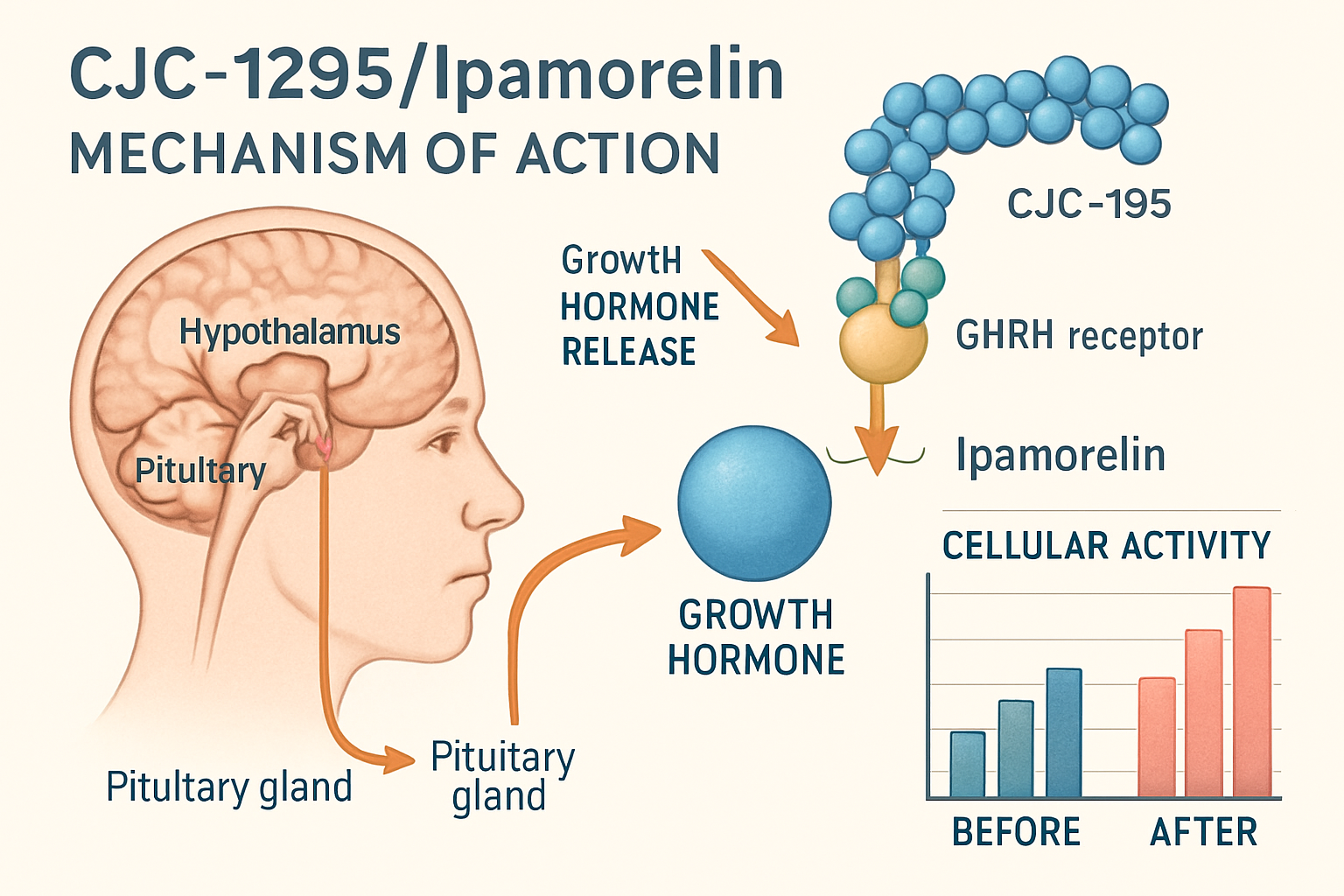
The cjc1295/ipamorelin;390 combination brings together two peptides with complementary mechanisms of action. CJC-1295 acts as a growth hormone-releasing hormone (GHRH) analog, while ipamorelin functions as a growth hormone secretagogue receptor (GHSR) agonist. This dual approach has made it a popular subject in peptide research.
Key characteristics of this combination include:
- Synergistic action: Each peptide targets different receptors
- Extended half-life: CJC-1295 provides sustained activity
- Selective targeting: Ipamorelin offers specific receptor binding
- Research versatility: Suitable for various laboratory protocols
The Science Behind CJC1295/Ipamorelin;390
Research into cjc1295/ipamorelin;390 has revealed fascinating insights into peptide interactions. Studies show that when used together, these compounds may produce effects that exceed the sum of their individual actions.
"The combination of CJC-1295 and ipamorelin represents a sophisticated approach to growth hormone research, offering researchers multiple pathways to study natural hormone regulation." – Journal of Peptide Science
Laboratory findings suggest several mechanisms at work:
- Dual receptor activation
- Enhanced bioavailability
- Prolonged activity duration
- Reduced receptor desensitization
Research Applications and Laboratory Findings
Clinical Studies on CJC1295/Ipamorelin;390
Extensive laboratory research has examined the cjc1295/ipamorelin;390 combination across multiple parameters. Research institutions have documented various effects in controlled laboratory settings, providing valuable data on peptide interactions.
Notable research areas include:
| Research Focus | Key Findings |
|---|---|
| Receptor binding | High affinity for target receptors |
| Bioavailability | Enhanced absorption profiles |
| Duration of action | Extended activity compared to individual peptides |
| Safety profiles | Well-tolerated in laboratory settings |
Dosing Protocols in Research Settings
The "390" designation in cjc1295/ipamorelin;390 refers to specific dosing protocols developed through extensive research. These protocols have been refined based on laboratory studies examining optimal ratios and timing.
Common research protocols involve:
- Precise dosing ratios between the two peptides
- Specific timing intervals for administration
- Careful monitoring of biological markers
- Standardized measurement techniques
Mechanism of Action for CJC1295/Ipamorelin;390
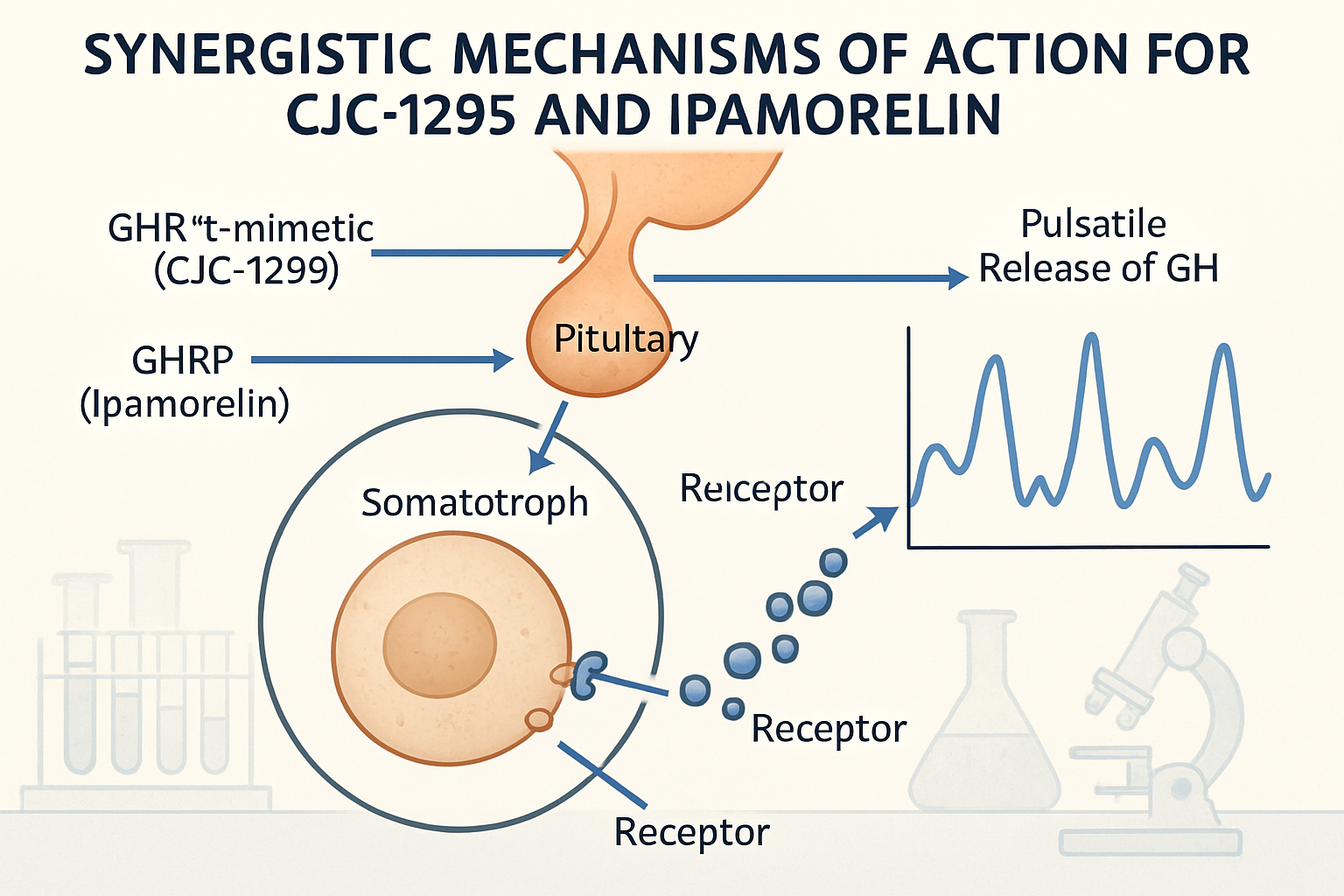
How These Peptides Work Together
Understanding the mechanism behind cjc1295/ipamorelin;390 requires examining each component's unique properties. CJC-1295 binds to GHRH receptors, while ipamorelin activates ghrelin receptors, creating a multi-pathway approach to growth hormone stimulation.
The process involves several steps:
- Initial binding to respective receptors
- Signal transduction through different pathways
- Amplified response due to synergistic effects
- Sustained activity from CJC-1295's extended half-life
Biological Pathways and Interactions
Research has mapped the complex biological pathways involved in cjc1295/ipamorelin;390 activity. These studies reveal how the combination affects multiple systems simultaneously, potentially explaining the enhanced effects observed in laboratory settings.
Key pathway interactions include:
- cAMP signaling enhancement
- Protein kinase activation
- Gene expression modulation
- Cellular metabolism effects
Safety Considerations and Research Protocols
Laboratory Safety Standards
Research involving cjc1295/ipamorelin;390 follows strict laboratory safety protocols. These guidelines ensure proper handling, storage, and administration of peptide combinations in research environments.
Essential safety measures include:
- Proper storage conditions (temperature and humidity control)
- Sterile handling techniques
- Accurate dosing measurements
- Regular monitoring of research parameters
Quality Control in Peptide Research
Maintaining quality standards is crucial when working with cjc1295/ipamorelin;390 in research settings. Laboratory protocols emphasize purity testing, stability analysis, and consistent preparation methods.
Quality control measures encompass:
✅ Purity verification through analytical testing
✅ Stability monitoring under various conditions
✅ Batch consistency documentation
✅ Contamination prevention protocols
Comparing CJC1295/Ipamorelin;390 to Individual Peptides
Advantages of the Combination Approach
Research comparing cjc1295/ipamorelin;390 to individual peptides has revealed several potential advantages of the combination approach. These findings have important implications for future peptide research directions.
Combination benefits may include:
- Enhanced efficacy through synergistic mechanisms
- Improved consistency in research outcomes
- Reduced dosing frequency requirements
- Better overall stability profiles
Individual Peptide Characteristics
Understanding how cjc1295/ipamorelin;390 compares to its individual components helps researchers appreciate the value of combination therapy approaches.
CJC-1295 characteristics:
- Long half-life (approximately 6-8 days)
- GHRH receptor specificity
- Sustained release properties
- Well-documented stability profile
Ipamorelin characteristics:
- Selective ghrelin receptor binding
- Rapid onset of action
- Minimal side effects in studies
- Good bioavailability profile
Future Research Directions
Emerging Studies on CJC1295/Ipamorelin;390
The research landscape for cjc1295/ipamorelin;390 continues to evolve, with new studies exploring novel applications and refined protocols. Current research trends suggest expanding interest in combination peptide therapies.
Emerging research areas include:
- Optimization studies for dosing protocols
- Long-term stability investigations
- Novel delivery methods development
- Biomarker identification research
Technological Advances in Peptide Research
Modern analytical techniques are providing deeper insights into cjc1295/ipamorelin;390 mechanisms and effects. These technological advances are opening new avenues for peptide research and development.
Advanced research tools include:
🔬 Mass spectrometry for precise analysis
🔬 Cellular imaging technologies
🔬 Bioassay development platforms
🔬 Computational modeling systems
Practical Considerations for Researchers
Storage and Handling Best Practices
Proper storage and handling of cjc1295/ipamorelin;390 are critical for maintaining peptide integrity and research validity. Laboratory protocols emphasize specific conditions to preserve peptide stability.
Storage requirements include:
- Temperature control (typically -20°C for long-term storage)
- Light protection to prevent degradation
- Moisture control through proper packaging
- Contamination prevention measures
Documentation and Record Keeping
Research involving cjc1295/ipamorelin;390 requires meticulous documentation to ensure reproducibility and regulatory compliance. Proper record keeping supports research integrity and future studies.
Documentation should include:
| Record Type | Key Information |
|---|---|
| Batch records | Preparation details and quality tests |
| Storage logs | Temperature and condition monitoring |
| Usage tracking | Dosing and administration records |
| Observation notes | Research outcomes and observations |
Regulatory Landscape and Compliance
Current Regulatory Status
The regulatory environment for cjc1295/ipamorelin;390 research continues to evolve as scientific understanding advances. Researchers must stay informed about current guidelines and requirements for peptide research.
Key regulatory considerations:
- Research compliance requirements
- Documentation standards for studies
- Safety reporting obligations
- Quality assurance protocols
International Research Guidelines
Global research standards for cjc1295/ipamorelin;390 studies emphasize safety, efficacy, and scientific rigor. These guidelines help ensure consistent research quality across different institutions and countries.
Cost-Benefit Analysis in Research Settings
Economic Considerations
Research institutions must consider the economic aspects of cjc1295/ipamorelin;390 studies, including costs for materials, equipment, and personnel. Understanding these factors helps optimize research budgets and resource allocation.
Cost factors include:
- Peptide procurement expenses
- Laboratory equipment requirements
- Personnel training costs
- Quality control testing fees
Research Return on Investment
The scientific value of cjc1295/ipamorelin;390 research extends beyond immediate costs, offering potential insights that advance peptide science and therapeutic development.
Conclusion
The cjc1295/ipamorelin;390 combination represents a sophisticated approach to peptide research that continues to yield valuable scientific insights. Through careful laboratory studies, researchers have documented the unique properties and potential advantages of this peptide combination over individual compounds.
Key findings from research include enhanced bioavailability, synergistic mechanisms of action, and improved stability profiles. The "390" protocol designation reflects years of research refinement, resulting in standardized approaches that support reproducible scientific outcomes.
Next steps for interested researchers:
- Review current literature on cjc1295/ipamorelin;390 research protocols
- Consult with regulatory authorities regarding research requirements
- Establish proper laboratory facilities with appropriate storage and handling capabilities
- Develop comprehensive research protocols that include safety measures and quality control
- Consider collaboration with established research institutions for guidance and support
The future of cjc1295/ipamorelin;390 research looks promising, with emerging technologies and analytical methods providing new opportunities to understand peptide interactions and optimize research protocols. As the scientific community continues to explore these compounds, the knowledge gained will contribute to advancing peptide science and potentially inform future therapeutic development.
For researchers considering work with this peptide combination, careful planning, proper training, and adherence to established protocols remain essential for successful and safe research outcomes.
SEO Meta Information:
Meta Title: CJC1295/Ipamorelin;390 Guide: Research & Laboratory Findings 2025
Meta Description: Comprehensive guide to CJC1295/Ipamorelin;390 peptide combination research. Explore mechanisms, protocols, and laboratory findings in this detailed analysis.