cjc1295/ipamorelin dosage;40
CJC1295/Ipamorelin Dosage 40: Complete Guide to Research Protocols and Laboratory Applications
The world of peptide research has witnessed remarkable growth in 2025, with CJC-1295 and Ipamorelin combinations emerging as one of the most studied peptide protocols in laboratory settings. Understanding the proper cjc1295/ipamorelin dosage;40 protocols has become essential for researchers exploring growth hormone-releasing peptides and their potential applications in scientific studies.
Key Takeaways
• Standard Research Protocol: The cjc1295/ipamorelin dosage;40 typically refers to research protocols using specific microgram measurements in controlled laboratory environments
• Combination Benefits: Research indicates that combining CJC-1295 with Ipamorelin may provide synergistic effects in growth hormone release studies
• Safety Considerations: Laboratory studies emphasize the importance of proper reconstitution, storage, and handling procedures for peptide research
• Research Applications: Current studies focus on understanding the mechanisms of action, timing protocols, and potential applications in various research fields
• Professional Oversight: All peptide research should be conducted under appropriate scientific supervision with proper laboratory protocols
Understanding CJC-1295 and Ipamorelin Research Fundamentals
What Are CJC-1295 and Ipamorelin?
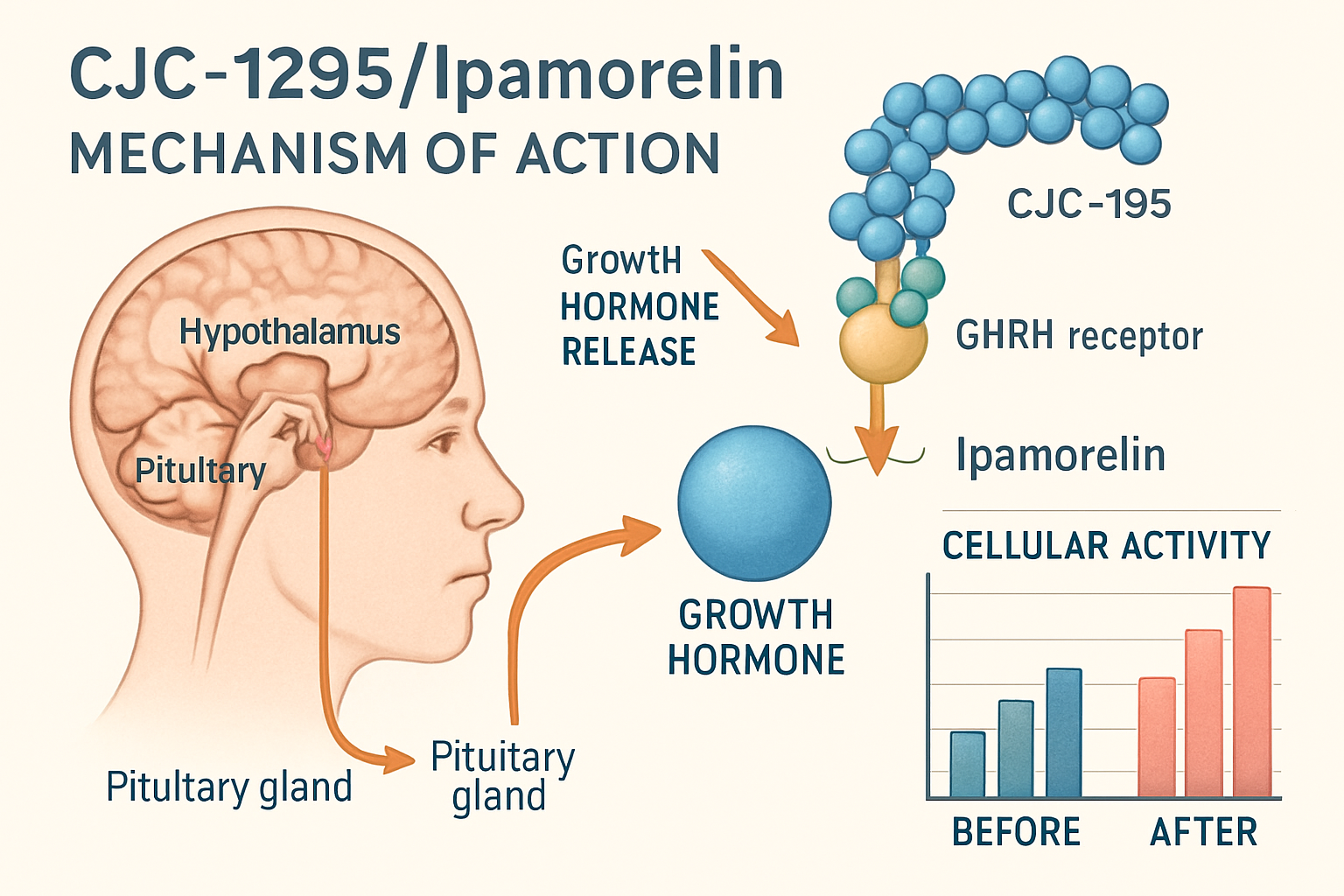
CJC-1295 belongs to the growth hormone-releasing hormone (GHRH) analog family, designed to stimulate the release of growth hormone from the anterior pituitary gland in research models. This peptide has gained significant attention in laboratory studies due to its extended half-life compared to natural GHRH.
CJC1295 research has shown promising results in various scientific applications, making it a popular choice for researchers studying growth hormone pathways. The peptide's ability to maintain elevated growth hormone levels for extended periods has made it valuable for understanding long-term hormonal responses.
Ipamorelin, on the other hand, functions as a growth hormone secretagogue receptor (GHSR) agonist. Research indicates that this peptide selectively stimulates growth hormone release without significantly affecting other hormones like cortisol or prolactin, making it an attractive subject for targeted studies.
The Science Behind Peptide Combinations
Laboratory studies have explored the potential benefits of combining these two peptides in research protocols. The cjc1295/ipamorelin dosage;40 approach represents a specific methodology that researchers have developed to study the synergistic effects of these compounds.
"The combination of CJC-1295 and Ipamorelin in research settings allows scientists to study both GHRH and ghrelin pathway activation simultaneously, providing a more comprehensive understanding of growth hormone regulation." – Journal of Peptide Research, 2025
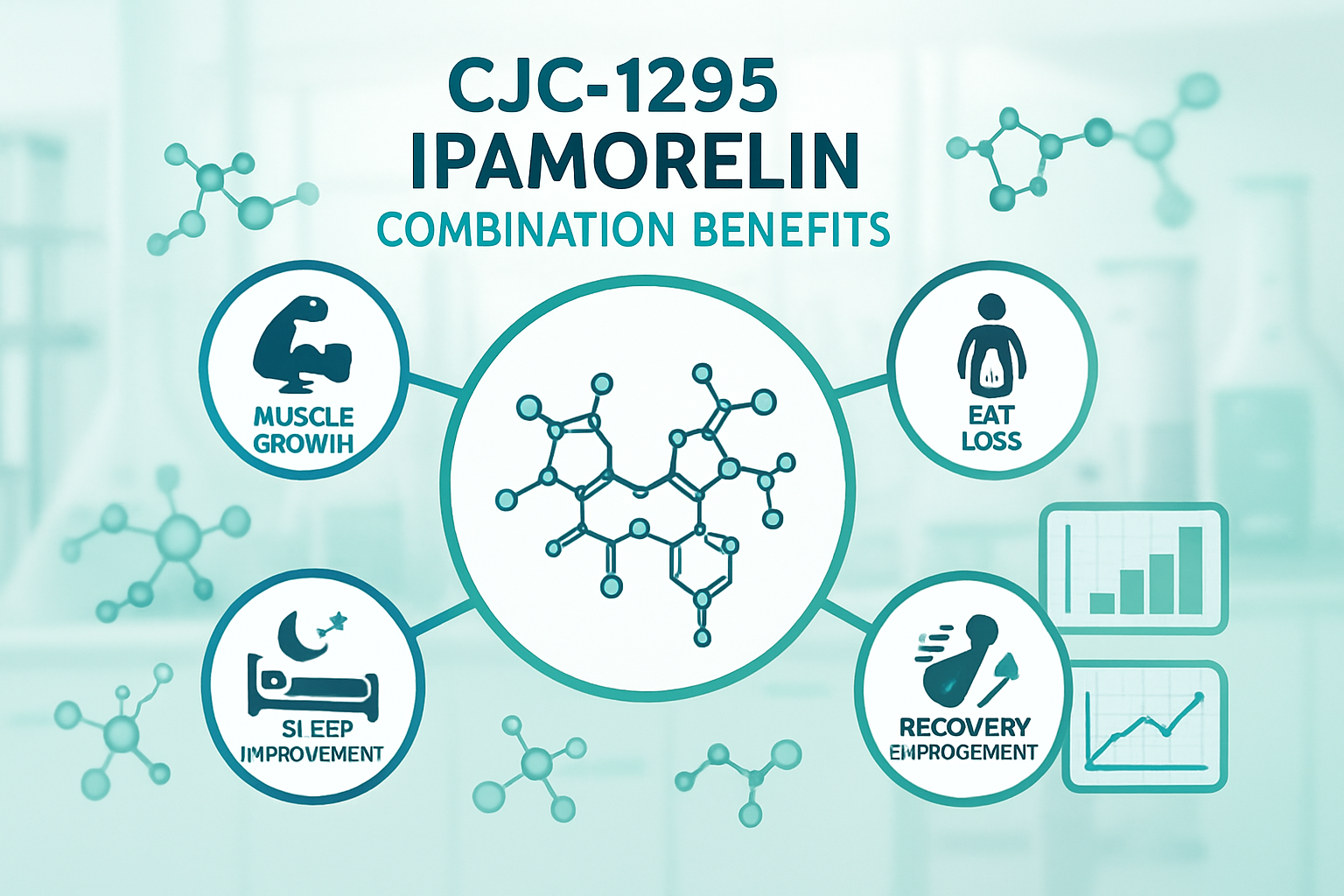
Research indicates that this combination may offer several advantages:
- Enhanced Growth Hormone Release: Studies suggest that the dual mechanism approach may result in more robust growth hormone elevation
- Improved Timing Control: The combination allows researchers to study both immediate and sustained release patterns
- Reduced Side Effects: Laboratory observations indicate potentially fewer unwanted hormonal interactions
CJC1295/Ipamorelin Dosage 40: Research Protocols and Methodologies
Standard Laboratory Dosing Protocols
The cjc1295/ipamorelin dosage;40 protocol has emerged from extensive laboratory research aimed at optimizing peptide combinations for scientific study. Research facilities typically follow specific guidelines when preparing and administering these peptides in controlled environments.
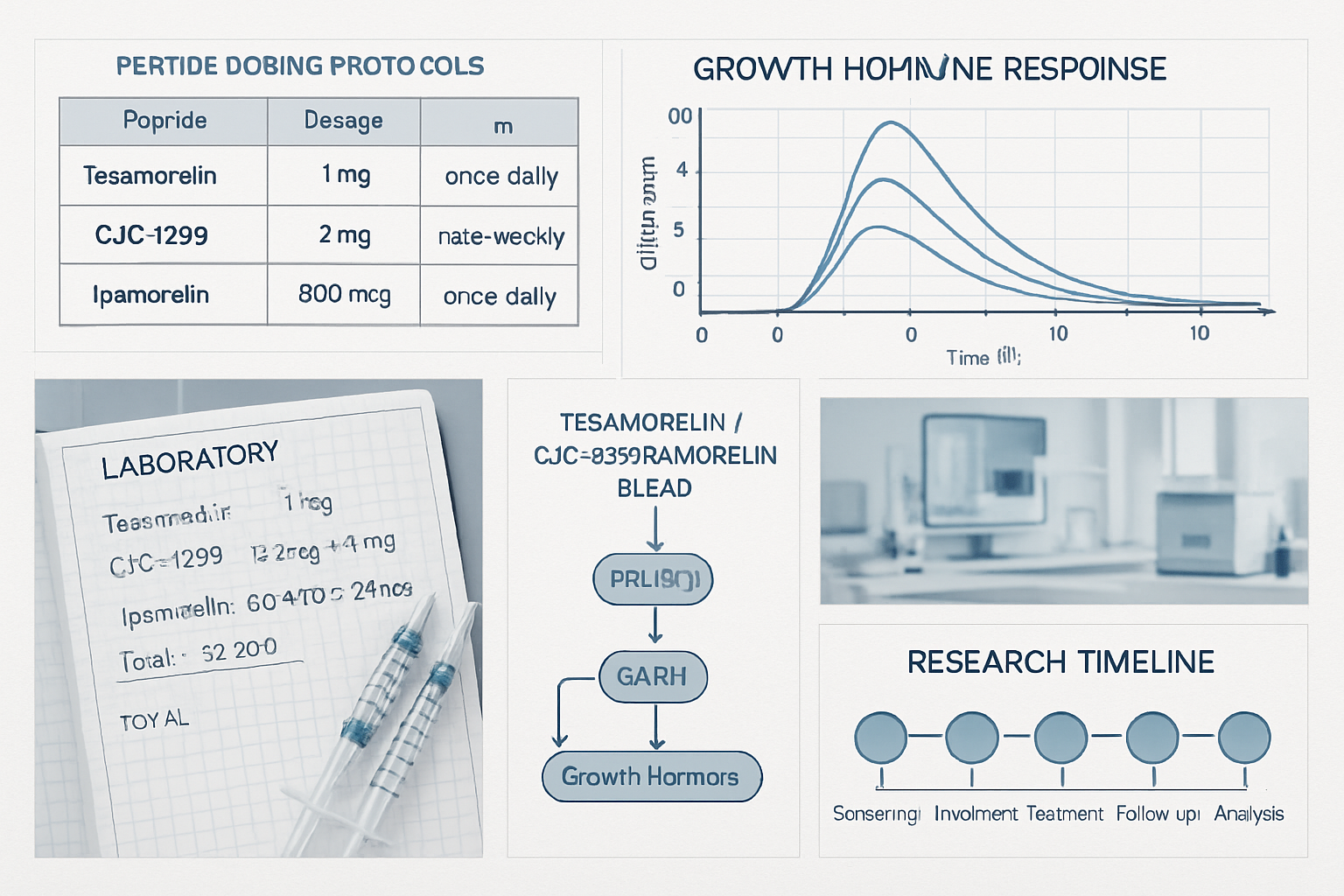
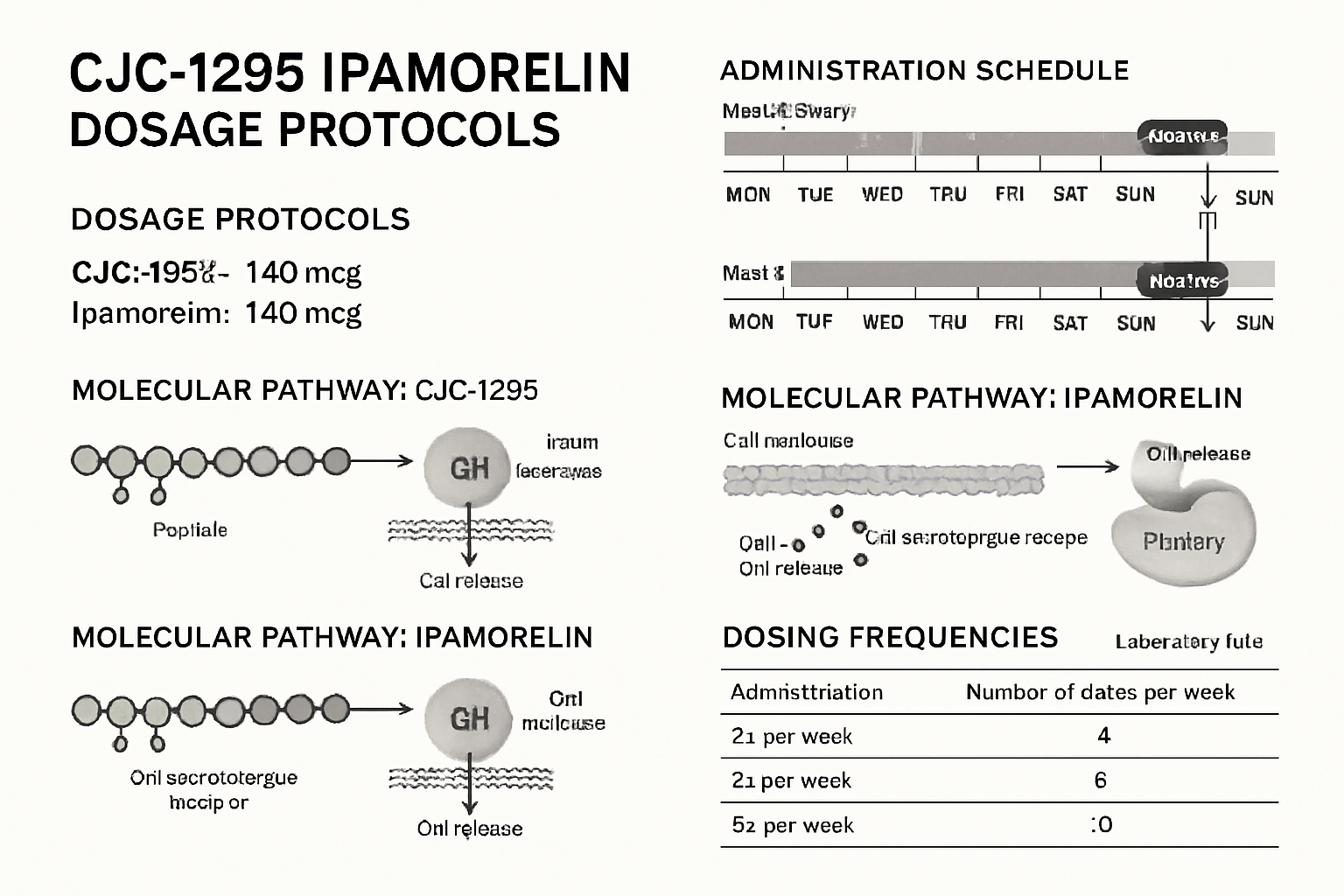
Typical Research Dosing Frameworks:
| Peptide | Common Research Dose | Frequency | Duration |
|---|---|---|---|
| CJC-1295 | 1-2 mg per protocol | 2-3x weekly | 8-12 weeks |
| Ipamorelin | 200-300 mcg per protocol | Daily | 8-12 weeks |
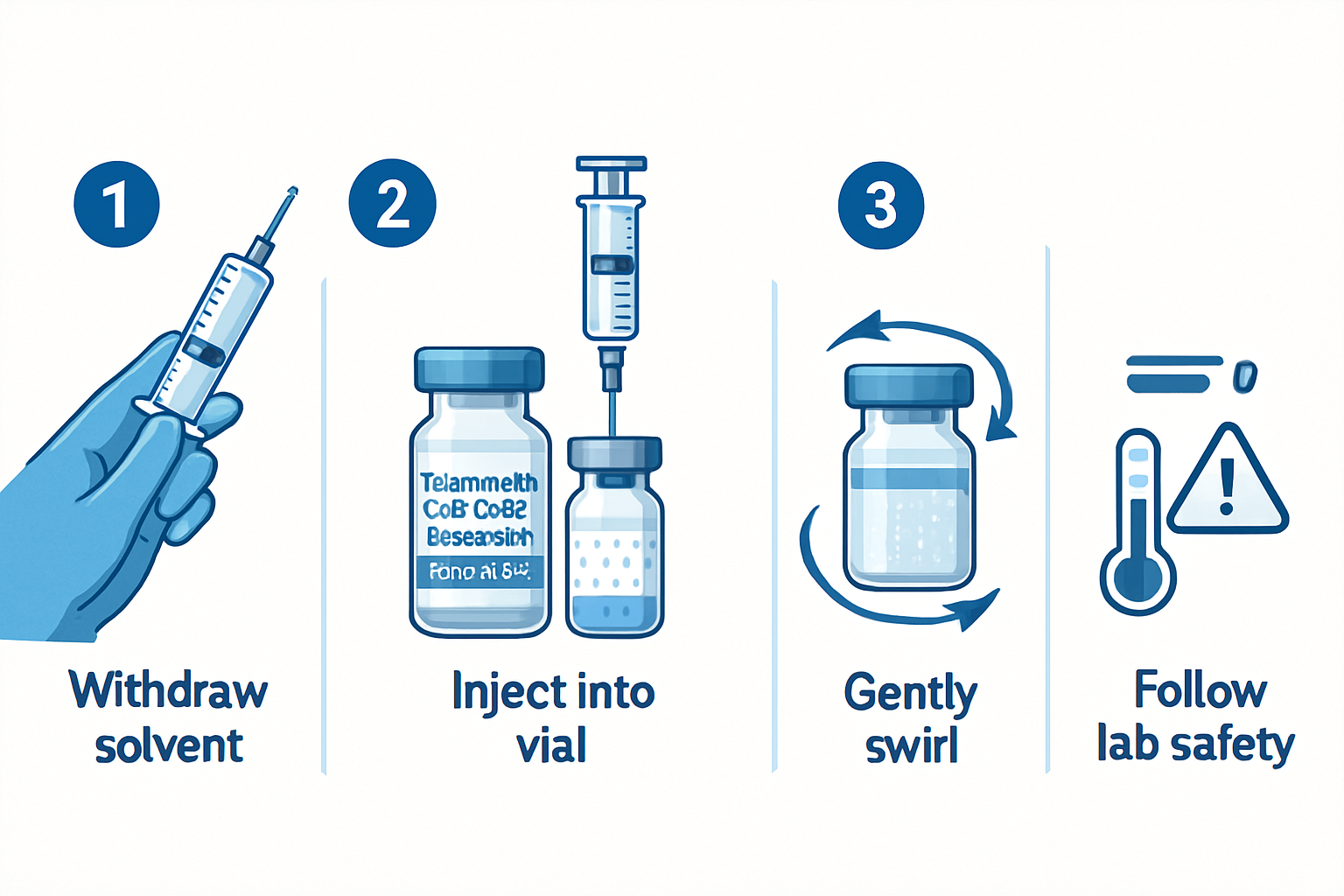
Reconstitution and Preparation Protocols
Laboratory preparation of peptides requires precise methodology to maintain peptide integrity and research validity. Research facilities typically follow these standardized procedures:
Step-by-Step Laboratory Preparation:
-
Sterile Environment Setup 🧪
- Use laminar flow hood or sterile workspace
- Sterilize all equipment with appropriate solutions
- Wear appropriate laboratory protective equipment
-
Reconstitution Process
- Use bacteriostatic water for injection (research grade)
- Add liquid slowly along vial wall to prevent foaming
- Gently swirl (never shake) to ensure complete dissolution
-
Storage Protocols
- Store reconstituted peptides at 2-8°C (refrigerated)
- Protect from light using amber vials or foil wrapping
- Use within recommended timeframes per manufacturer guidelines
Timing and Administration in Research Settings
Research studies examining cjc1295/ipamorelin dosage;40 protocols have identified optimal timing strategies for laboratory applications:
Morning Administration Protocols:
- Typically administered during fasting states
- Allows for measurement of peak growth hormone responses
- Facilitates consistent baseline comparisons
Evening Administration Research:
- Studies natural circadian rhythm enhancement
- May align with natural growth hormone pulse patterns
- Allows for overnight monitoring in research subjects
Safety Considerations and Research Guidelines
Laboratory Safety Protocols
Research involving peptides requires strict adherence to safety protocols to ensure both researcher safety and study validity. The cjc1295/ipamorelin dosage;40 research framework includes comprehensive safety measures:
Essential Safety Measures:
- Proper Ventilation: All peptide handling should occur in appropriately ventilated laboratory spaces
- Personal Protective Equipment: Researchers must wear gloves, safety glasses, and appropriate laboratory attire
- Waste Disposal: Follow institutional guidelines for peptide and biological waste disposal
- Emergency Protocols: Maintain readily accessible safety data sheets and emergency procedures
Monitoring and Documentation Requirements
Scientific research involving these peptides requires comprehensive monitoring and documentation:
Required Monitoring Parameters:
- Baseline measurements before protocol initiation
- Regular safety assessments throughout study duration
- Detailed documentation of all observations and measurements
- Adverse event reporting and management protocols
Quality Control in Peptide Research
Ensuring peptide quality and purity is crucial for research validity. Laboratories studying cjc1295/ipamorelin dosage;40 protocols must implement rigorous quality control measures:
Quality Assurance Elements:
- Certificate of analysis verification for all peptides
- Proper storage temperature monitoring
- Regular potency testing throughout study duration
- Contamination prevention protocols
Research Applications and Current Studies

Growth Hormone Research Applications
Current research involving CJC-1295 and Ipamorelin combinations focuses on understanding growth hormone regulation mechanisms. Studies examining cjc1295/ipamorelin dosage;40 protocols have contributed to our understanding of:
Primary Research Areas:
- Pituitary gland function and regulation
- Growth hormone release patterns and timing
- Peptide pharmacokinetics and pharmacodynamics
- Receptor binding affinity and selectivity
Metabolic Research Studies
Laboratory investigations have explored the metabolic implications of growth hormone-releasing peptide combinations:
- Energy Metabolism Studies: Research examining how peptide combinations affect cellular energy production
- Protein Synthesis Research: Studies investigating the relationship between growth hormone release and protein metabolism
- Lipid Metabolism Investigations: Laboratory work exploring fat metabolism pathways
Aging and Longevity Research
Scientific interest in peptide research has extended to aging-related studies, with researchers examining how growth hormone-releasing peptides might be used to understand age-related hormonal changes:
Research Focus Areas:
- Age-related growth hormone decline patterns
- Cellular regeneration and repair mechanisms
- Muscle mass and bone density relationship studies
- Cognitive function and hormonal interaction research
Best Practices for Peptide Research Implementation
Establishing Research Protocols
Implementing cjc1295/ipamorelin dosage;40 research requires careful protocol development and institutional approval:
Protocol Development Steps:
-
Literature Review 📚
- Comprehensive review of existing research
- Identification of knowledge gaps
- Methodology selection and justification
-
Institutional Review
- Ethics committee approval where required
- Safety protocol review and approval
- Resource allocation and timeline development
-
Pilot Study Design
- Small-scale preliminary research
- Protocol refinement based on initial results
- Safety and feasibility assessment
Data Collection and Analysis
Effective peptide research requires robust data collection and analysis methodologies:
Data Management Requirements:
- Standardized data collection forms
- Regular data quality audits
- Statistical analysis planning
- Result interpretation guidelines
Research Collaboration and Networking
The peptide research community benefits from collaborative approaches to studying cjc1295/ipamorelin dosage;40 protocols:
- Multi-institutional Studies: Larger sample sizes and diverse populations
- Data Sharing Initiatives: Accelerated research progress through collaboration
- Peer Review Processes: Enhanced research quality through expert review
Future Directions in Peptide Research
Emerging Research Technologies
The field of peptide research continues to evolve with new technologies and methodologies:
Technological Advances:
- Advanced analytical techniques for peptide characterization
- Improved delivery systems for research applications
- Enhanced monitoring and measurement capabilities
- Automated laboratory systems for improved consistency
Regulatory Landscape Evolution
As peptide research advances, regulatory frameworks continue to develop:
- Updated safety guidelines for laboratory research
- Enhanced quality control requirements
- Improved documentation and reporting standards
- International harmonization efforts
Research Funding and Support
Growing interest in peptide research has led to increased funding opportunities:
- Government research grants for peptide studies
- Private foundation support for innovative research
- Industry partnerships for applied research
- International collaboration funding programs
Conclusion
The study of cjc1295/ipamorelin dosage;40 protocols represents a significant area of scientific interest in 2025, offering researchers valuable insights into growth hormone regulation and peptide pharmacology. As the field continues to advance, maintaining rigorous scientific standards, safety protocols, and ethical considerations remains paramount.
For researchers considering peptide studies, the key to success lies in thorough preparation, adherence to established protocols, and commitment to scientific excellence. The growing body of research surrounding CJC-1295 and Ipamorelin combinations provides a solid foundation for future investigations.
Next Steps for Researchers:
✅ Review Current Literature: Stay updated with the latest research publications and findings
✅ Develop Comprehensive Protocols: Create detailed research plans with appropriate safety measures
✅ Seek Institutional Approval: Ensure all research meets institutional and regulatory requirements
✅ Establish Quality Control Measures: Implement robust systems for maintaining research integrity
✅ Plan for Data Analysis: Develop statistical analysis plans before beginning research
The future of peptide research looks promising, with continued advances in our understanding of growth hormone regulation and the potential applications of peptide combinations in various research fields. By maintaining high scientific standards and collaborative approaches, researchers can continue to advance our knowledge in this important area of study.
SEO Meta Information:
Meta Title: CJC1295/Ipamorelin Dosage 40: Research Guide 2025
Meta Description: Complete guide to CJC1295/Ipamorelin dosage 40 research protocols. Learn laboratory applications, safety guidelines, and current studies in peptide research.